Cancer Res Treat.
2022 Jan;54(1):157-173. 10.4143/crt.2020.1063.
Potentiation of the Anticancer Effects by Combining Docetaxel with Ku-0063794 against Triple-Negative Breast Cancer Cells
- Affiliations
-
- 1Department of Surgery, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 2Catholic Central Laboratory of Surgery, Institute of Biomedical Industry, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2524597
- DOI: http://doi.org/10.4143/crt.2020.1063
Abstract
- Purpose
mTORC1 and mTORC2 inhibition by Ku-0063794 could confer profound anticancer effects against cancer cells because it eliminates feedback activation of Akt. Herein, we aimed to determine anticancer effects of docetaxel and Ku-0063794, individually or in combination, against breast cancer cells, especially triple-negative breast cancer (TNBC) cells.
Materials and Methods
MCF-7 breast cancer and MDA-MB-231 TNBC cell lines for in vitro studies and mouse xenograft model for in vivo studies were used to investigate the effect of docetaxel, Ku-0063794, or their combination.
Results
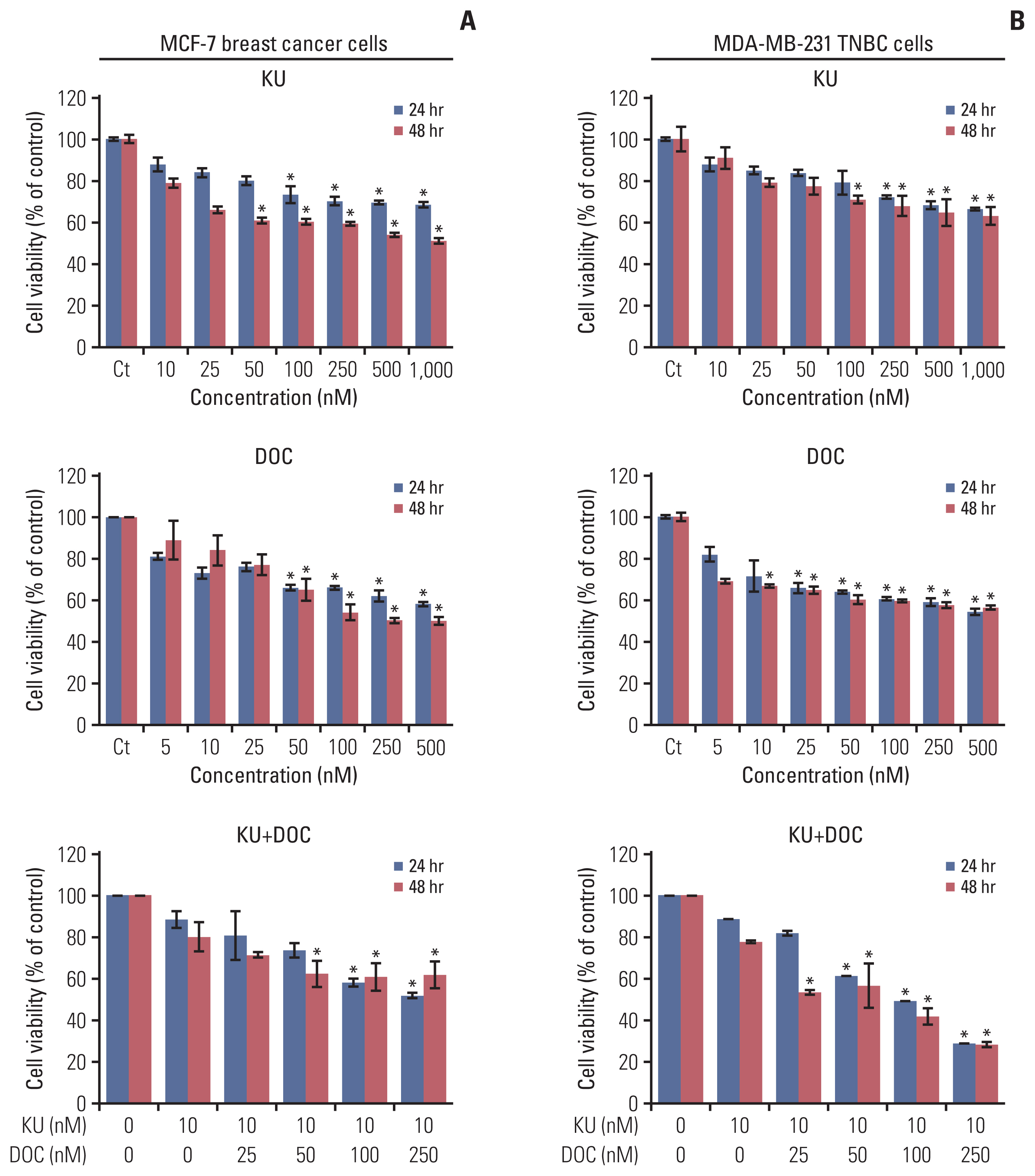
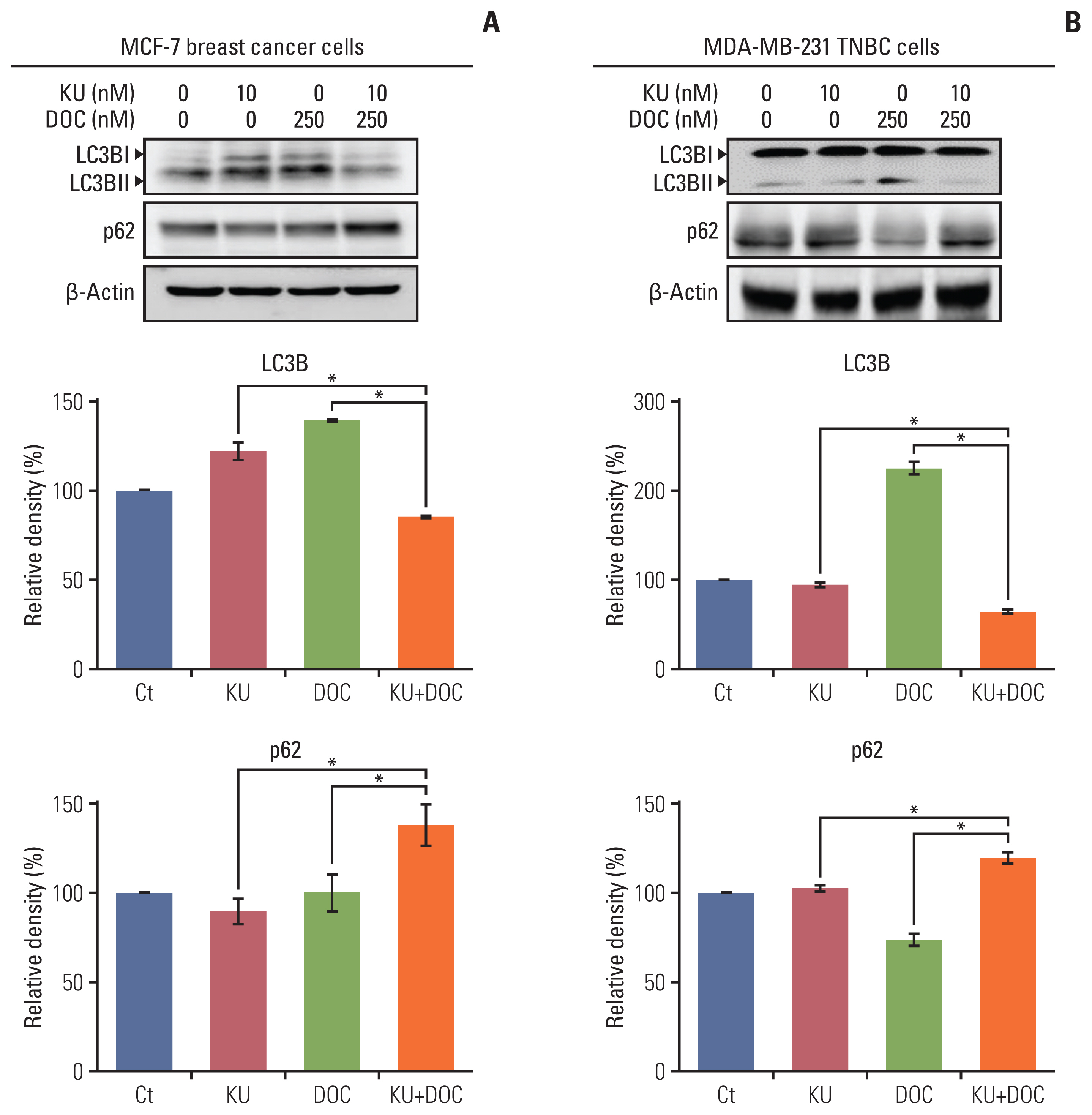
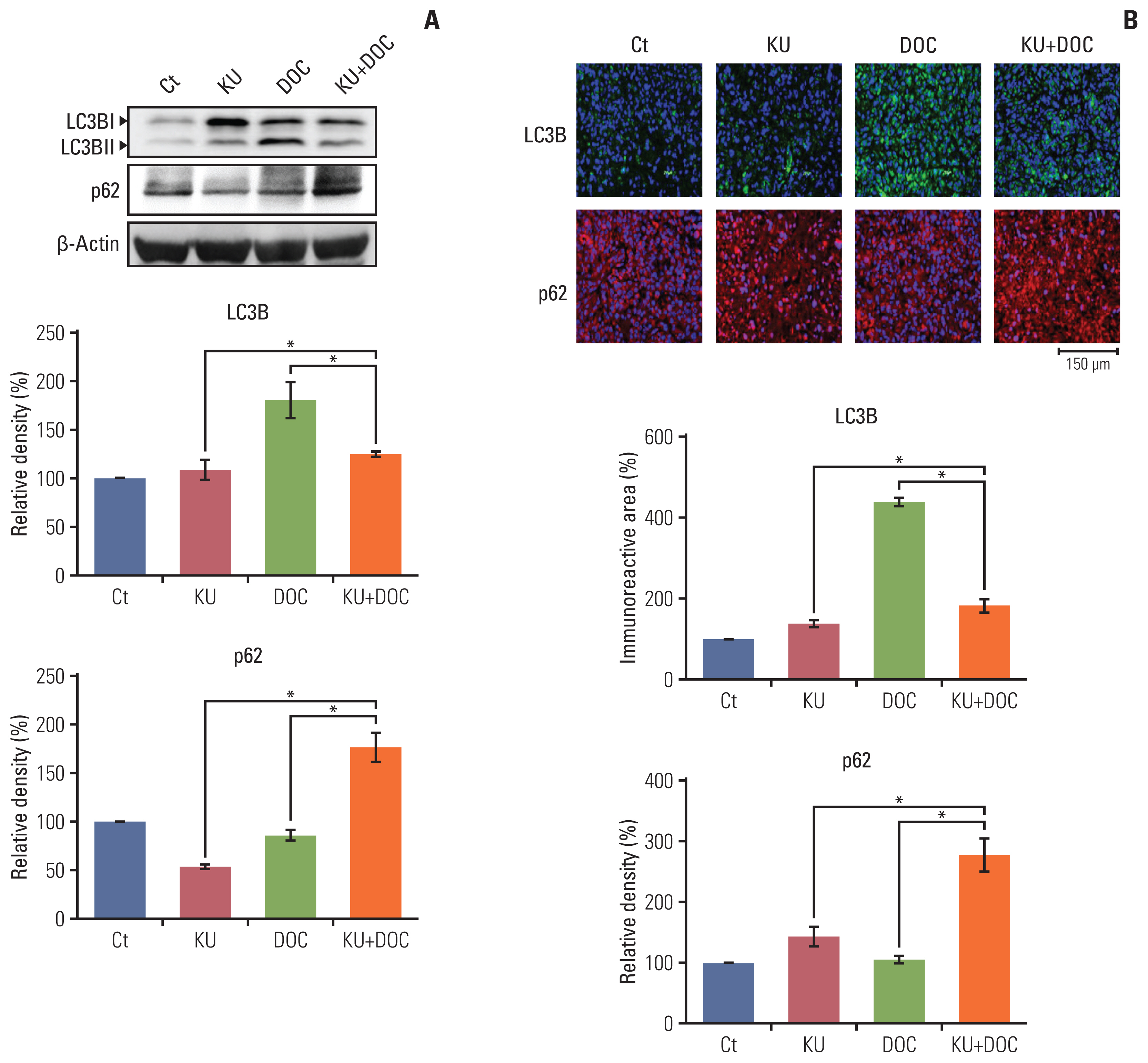
In the in vitro experiments, combination therapy synergistically reduced cell viability and induced higher apoptotic cell death in breast cancer cells than the individual monotherapies (p < 0.05). Western blot analysis and flow cytometric analysis showed that the combination therapy induced higher apoptotic cell death than the individual monotherapies (p < 0.05). In the in vivo experiment, docetaxel and Ku-0063794 combination therapy reduced the growth of MDA-MB-231 cells xenografted in the nude mice better than in the individual monotherapies (p < 0.05). Immunohistochemistry showed that the combination therapy induced the highest expression of cleaved caspase-3 and the lowest expression of Bcl-xL in the MDA-MB-231 cells xenografted in the nude mice (p < 0.05). Western blot analysis and immunofluorescence, incorporating both in vitro and in vivo experiments, consistently validated that unlike individual monotherapies, docetaxel and Ku-0063794 combination therapy significantly inhibited epithelial-mesenchymal transition (EMT) and autophagy (p < 0.05).
Conclusion
These data suggest that docetaxel and Ku-0063794 combination therapy has higher anticancer activities over individual monotherapies against MDA-MB-231 TNBC cells through a greater inhibition of autophagy and EMT.
Keyword
Figure
Reference
-
References
1. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007; 109:1721–8.2. Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008; 52:108–18.
Article3. Dreyer G, Vandorpe T, Smeets A, Forceville K, Brouwers B, Neven P, et al. Triple negative breast cancer: clinical characteristics in the different histological subtypes. Breast. 2013; 22:761–6.
Article4. Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009; 9:29–33.
Article5. Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008; 113:2638–45.6. Jhan JR, Andrechek ER. Triple-negative breast cancer and the potential for targeted therapy. Pharmacogenomics. 2017; 18:1595–609.
Article7. Murtagh G, Lyons T, O’Connell E, Ballot J, Geraghty L, Fennelly D, et al. Late cardiac effects of chemotherapy in breast cancer survivors treated with adjuvant doxorubicin: 10-year follow-up. Breast Cancer Res Treat. 2016; 156:501–6.
Article8. Garcia-Martinez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, et al. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR). Biochem J. 2009; 421:29–42.9. Ali S, Moreau A, Melchiorri D, Camarero J, Josephson F, Olimpier O, et al. Blinatumomab for acute lymphoblastic leukemia: the first bispecific T-cell engager antibody to be approved by the EMA for minimal residual disease. Oncologist. 2020; 25:e709–15.
Article10. Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005; 17:596–603.
Article11. Xue M, Thompson P, Kelso I, Jackson C. Activated protein C stimulates proliferation, migration and wound closure, inhibits apoptosis and upregulates MMP-2 activity in cultured human keratinocytes. Exp Cell Res. 2004; 299:119–27.
Article12. Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010; 29:3733–44.
Article13. Jovanovic B, Mayer IA, Mayer EL, Abramson VG, Bardia A, Sanders ME, et al. A randomized phase II neoadjuvant study of cisplatin, paclitaxel with or without everolimus in patients with stage II/III triple-negative breast cancer (TNBC): responses and long-term outcome correlated with increased frequency of DNA damage response gene mutations, TNBC subtype, AR status, and Ki67. Clin Cancer Res. 2017; 23:4035–45.
Article14. Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009; 7:e38.
Article15. Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009; 10:307–18.
Article16. Takebe N, Warren RQ, Ivy SP. Breast cancer growth and metastasis: interplay between cancer stem cells, embryonic signaling pathways and epithelial-to-mesenchymal transition. Breast Cancer Res. 2011; 13:211.
Article17. Foroni C, Broggini M, Generali D, Damia G. Epithelial-mesenchymal transition and breast cancer: role, molecular mechanisms and clinical impact. Cancer Treat Rev. 2012; 38:689–97.
Article18. Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003; 5:R217–22.
Article19. Cristofani R, Montagnani Marelli M, Cicardi ME, Fontana F, Marzagalli M, Limonta P, et al. Dual role of autophagy on docetaxel-sensitivity in prostate cancer cells. Cell Death Dis. 2018; 9:889.
Article20. Zhang J, Wang J, Wong YK, Sun X, Chen Y, Wang L, et al. Docetaxel enhances lysosomal function through TFEB activation. Cell Death Dis. 2018; 9:614.
Article21. Kim JO, Kim KH, Song IS, Cheon KS, Kim OH, Lee SC, et al. Potentiation of the anticancer effects of everolimus using a dual mTORC1/2 inhibitor in hepatocellular carcinoma cells. Oncotarget. 2017; 8:2936–48.
Article22. Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007; 27:19–40.
Article23. Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004; 6:1221–8.
Article24. Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015; 125:25–32.
Article25. Hu F, Zhao Y, Yu Y, Fang JM, Cui R, Liu ZQ, et al. Docetaxel-mediated autophagy promotes chemoresistance in castration-resistant prostate cancer cells by inhibiting STAT3. Cancer Lett. 2018; 416:24–30.
Article26. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008; 105:3374–9.
Article27. Lee SC, Kim KH, Kim OH, Lee SK, Hong HE, Choi BJ, et al. Everolimus plus Ku0063794 regimen promotes anticancer effects against hepatocellular carcinoma cells through the paradoxical inhibition of autophagy. Cancer Res Treat. 2018; 50:1023–38.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Caveolin-1 Modulates Docetaxel-Induced Cell Death in Breast Cancer Cell Subtypes through Different Mechanisms
- Clinicopathologic Characteristics and Prognosis of Early Stage Triple Negative Breast Cancer: Comparison with Non-triple Negative Group
- Docetaxel-loaded PLGA nanoparticles to increase pharmacological sensitivity in MDA-MB-231 and MCF-7 breast cancer cells
- Fear of Cancer Recurrence and Unmet Needs in Triple Negative Breast Cancer Survivors
- Bilateral Triple Negative Invasive Ductal Breast Carcinoma in a BRCA1 Mutation Carrier with Discrepant Pathologic Response to Neoadjuvant Chemotherapy