J Clin Neurol.
2019 Apr;15(2):175-183. 10.3988/jcn.2019.15.2.175.
A New Metabolic Network Correlated with Olfactory and Executive Dysfunctions in Idiopathic Rapid Eye Movement Sleep Behavior Disorder
- Affiliations
-
- 1Department of Nuclear Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea. yk3181@snu.ac.kr
- 2Department of Nuclear Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul, Korea.
- 4Department of Neurology, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea. wieber04@snu.ac.kr
- 5Department of Neurology, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Neurology, Seoul National University Hospital, Seoul, Korea.
- KMID: 2467731
- DOI: http://doi.org/10.3988/jcn.2019.15.2.175
Abstract
- BACKGROUND AND PURPOSE
To identify a metabolic network reflecting neurodegeneration in patients with idiopathic rapid eye movement (REM) sleep behavior disorder (iRBD).
METHODS
We recruited a prospective cohort comprising patients with de novo Parkinson's disease (PD) with probable REM sleep behavior disorder (PDRBD, n=21), polysomnography-confirmed iRBD patients (n=28), and age-matched healthy controls (HC) (n=24). PDRBD-related spatial covariance pattern (PDRBD-RP) were determined from 18F-fluorodeoxyglucose PET images of the PDRBD group and validated by reproduction in a separate PD cohort with polysomnography-confirmed REM sleep behavior disorder (n=11). We also confirmed via 18F-N-3-fluoropropyl-2β-carboxymethoxy-3β-(4-iodophenyl)-nortropane PET that none of our iRBD patients had any loss of dopamine transporters (DATs) suggestive of PD. Differences in the PDRBD-RP across groups were compared, and the clinical significance of these metabolic patterns in iRBD patients was further evaluated based on relationships with olfactory and cognitive functions, and striatal DAT densities.
RESULTS
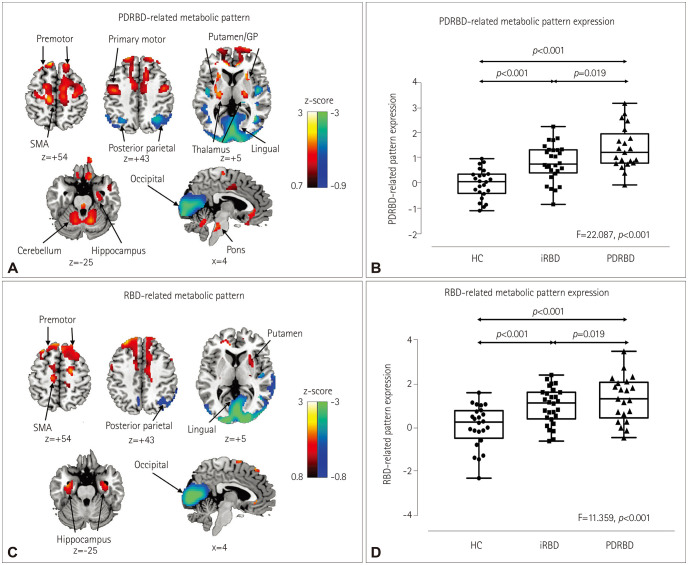
The PDRBD-RP reflected the previously reported PD-related covariance pattern and additionally showed relative metabolic increases in the hippocampus and premotor cortex. The PDRBD-RP gradually increased from the HC to iRBD patients and to the de novo and validation PDRBD groups. In iRBD patients, the PDRBD-RP was negatively correlated with olfactory and frontal executive functions (age-controlled p<0.01 for both), and tended to be negatively correlated with the striatal DAT density, although this was insignificant after age adjustment. During the mean follow-up period of 3.5 years, 5 of 11 iRBD patients with PDRBD-RP elevation had developed Lewy body diseases, whereas those without PDRBD-RP elevation had not.
CONCLUSIONS
Our results suggest that PDRBD-RP is an effective biomarker for monitoring the progression to neurodegenerative disease in iRBD patients.
Keyword
MeSH Terms
-
Cognition
Cohort Studies
Dopamine
Executive Function
Follow-Up Studies
Functional Neuroimaging
Hippocampus
Humans
Lewy Bodies
Metabolic Networks and Pathways*
Motor Cortex
Neurodegenerative Diseases
Parkinson Disease
Positron-Emission Tomography
Prospective Studies
REM Sleep Behavior Disorder*
Reproduction
Sleep, REM*
Smell
Dopamine
Figure
Reference
-
1. Boeve BF. Idiopathic REM sleep behaviour disorder in the development of Parkinson’s disease. Lancet Neurol. 2013; 12:469–482. PMID: 23578773.
Article2. Eckert T, Van Laere K, Tang C, Lewis DE, Edwards C, Santens P, et al. Quantification of Parkinson’s disease-related network expression with ECD SPECT. Eur J Nucl Med Mol Imaging. 2007; 34:496–501. PMID: 17096095.
Article3. Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson’s disease: test-retest reproducibility. J Cereb Blood Flow Metab. 2007; 27:597–605. PMID: 16804550.
Article4. Wu P, Yu H, Peng S, Dauvilliers Y, Wang J, Ge J, et al. Consistent abnormalities in metabolic network activity in idiopathic rapid eye movement sleep behaviour disorder. Brain. 2014; 137:3122–3128. PMID: 25338949.
Article5. Meles SK, Vadasz D, Renken RJ, Sittig-Wiegand E, Mayer G, Depboylu C, et al. FDG PET, dopamine transporter SPECT, and olfaction: combining biomarkers in REM sleep behavior disorder. Mov Disord. 2017; 32:1482–1486. PMID: 28734065.
Article6. Holtbernd F, Gagnon JF, Postuma RB, Ma Y, Tang CC, Feigin A, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology. 2014; 82:620–627. PMID: 24453082.
Article7. Meles SK, Renken RJ, Janzen A, Vadasz D, Pagani M, Arnaldi D, et al. The metabolic pattern of idiopathic REM sleep behavior disorder reflects early-stage Parkinson’s disease. J Nucl Med. 2018; 59:1437–1444. PMID: 29476004.8. Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson’s disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008; 79:1117–1121. PMID: 18682443.
Article9. Postuma RB, Adler CH, Dugger BN, Hentz JG, Shill HA, Driver-Dunckley E, et al. REM sleep behavior disorder and neuropathology in Parkinson’s disease. Mov Disord. 2015; 30:1413–1417. PMID: 26265105.
Article10. Lim JS, Shin SA, Lee JY, Nam H, Lee JY, Kim YK. Neural substrates of rapid eye movement sleep behavior disorder in Parkinson’s disease. Parkinsonism Relat Disord. 2016; 23:31–36. PMID: 26678512.
Article11. Li D, Huang P, Zang Y, Lou Y, Cen Z, Gu Q, et al. Abnormal baseline brain activity in Parkinson’s disease with and without REM sleep behavior disorder: a resting-state functional MRI study. J Magn Reson Imaging. 2017; 46:697–703. PMID: 27880010.
Article12. Stiasny-Kolster K, Mayer G, Schäfer S, Möller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire--a new diagnostic instrument. Mov Disord. 2007; 22:2386–2393. PMID: 17894337.
Article13. American Academy of Sleep Medicine. The International Classification of Sleep Disorder: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine;2005.14. Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope. 1996; 106:353–356. PMID: 8614203.15. Kim BG, Oh JH, Choi HN, Park SY. Simple assessment of olfaction in patients with chronic rhinosinusitis. Acta Otolaryngol. 2015; 135:258–263. PMID: 25625195.
Article16. Kang Y, Na DL. Seoul Neuropsychological Screening Battery. Seoul: Human Brain Research & Consulting Co.;2003.17. Habeck C, Foster NL, Perneczky R, Kurz A, Alexopoulos P, Koeppe RA, et al. Multivariate and univariate neuroimaging biomarkers of Alzheimer’s disease. Neuroimage. 2008; 40:1503–1515. PMID: 18343688.
Article18. Dang-Vu TT, Gagnon JF, Vendette M, Soucy JP, Postuma RB, Montplaisir J. Hippocampal perfusion predicts impending neurodegeneration in REM sleep behavior disorder. Neurology. 2012; 79:2302–2306. PMID: 23115214.
Article19. Calabresi P, Castrioto A, Di Filippo M, Picconi B. New experimental and clinical links between the hippocampus and the dopaminergic system in Parkinson’s disease. Lancet Neurol. 2013; 12:811–821. PMID: 23867199.
Article20. Ma Y, Tang C, Moeller JR, Eidelberg D. Abnormal regional brain function in Parkinson’s disease: truth or fiction? Neuroimage. 2009; 45:260–266. PMID: 18992824.
Article21. Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, et al. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain. 2000; 123:394–403. PMID: 10648446.22. Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012; 8:329–339. PMID: 22584158.
Article23. Fantini ML, Postuma RB, Montplaisir J, Ferini-Strambi L. Olfactory deficit in idiopathic rapid eye movements sleep behavior disorder. Brain Res Bull. 2006; 70:386–390. PMID: 17027774.
Article24. Mahlknecht P, Iranzo A, Högl B, Frauscher B, Müller C, Santamaría J, et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology. 2015; 84:654–658. PMID: 25609758.
Article25. Massicotte-Marquez J, Décary A, Gagnon JF, Vendette M, Mathieu A, Postuma RB, et al. Executive dysfunction and memory impairment in idiopathic REM sleep behavior disorder. Neurology. 2008; 70:1250–1257. PMID: 18216303.
Article26. Gagnon JF, Vendette M, Postuma RB, Desjardins C, Massicotte-Marquez J, Panisset M, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson’s disease. Ann Neurol. 2009; 66:39–47. PMID: 19670440.
Article27. Rodrigues Brazète J, Montplaisir J, Petit D, Postuma RB, Bertrand JA, Génier Marchand D, et al. Electroencephalogram slowing in rapid eye movement sleep behavior disorder is associated with mild cognitive impairment. Sleep Med. 2013; 14:1059–1063. PMID: 24095264.
Article28. Vendette M, Montplaisir J, Gosselin N, Soucy JP, Postuma RB, Dang-Vu TT, et al. Brain perfusion anomalies in rapid eye movement sleep behavior disorder with mild cognitive impairment. Mov Disord. 2012; 27:1255–1261. PMID: 22791632.
Article29. Génier Marchand D, Montplaisir J, Postuma RB, Rahayel S, Gagnon JF. Detecting the cognitive prodrome of dementia with Lewy bodies: a prospective study of REM sleep behavior disorder. Sleep. 2017; 40:zsw014.
Article30. Tröster AI. Neuropsychological characteristics of dementia with Lewy bodies and Parkinson’s disease with dementia: differentiation, early detection, and implications for “mild cognitive impairment” and biomarkers. Neuropsychol Rev. 2008; 18:103–119. PMID: 18322801.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Seizure Like Movement During Rapid Eye Movement Sleep Diagnosed With Pseudo-Rapid Eye Movement Sleep Behavior Disorder Associated With Severe Obstructive Sleep Apnea
- Rapid Eye Movement Sleep Behavior Disorder
- Three Cases of Rapid Eye Movement Sleep Behavior Disorder after Encephalitis
- The Diagnosis and Management for REM Sleep Behavior Disorder
- Idiopathic REM Sleep Behavior Disorder in Young Adults and Quantitative Analysis of Polysomnography