J Nutr Health.
2019 Feb;52(1):26-35. 10.4163/jnh.2019.52.1.26.
Antioxidant activity of ethanol extract of Lycium barbarum's leaf with removal of chlorophyll
- Affiliations
-
- 1Department of Food and Nutrition, Chungam National University, Daejeon 34134, Korea. sunly@cnu.ac.kr
- 2Convergence Research Center for Natural Products, Chungnam National University, Daejeon 34134, Korea.
- KMID: 2466680
- DOI: http://doi.org/10.4163/jnh.2019.52.1.26
Abstract
- PURPOSE
The aim of this study was to estimate the antioxidant activities of 50%, 70%, and 100% ethanol extracts of Lycium barbarum leaf and chlorophyll removal extract.
METHODS
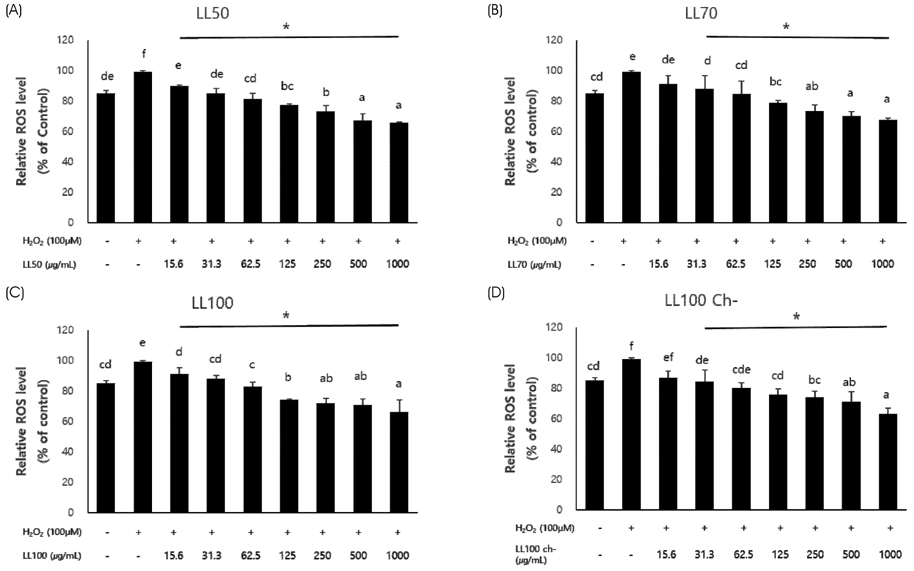
The antioxidant activities were estimated by measuring total polyphenol content and by assays of 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2"²-azino-bis (3-ethylbenzothiazoline-6-sulfate) (ABTS) radical scavenging activities and ferric reducing antioxidant power (FRAP). In addition, reactive oxygen species (ROS) production, DNA fragmentation, and antioxidant enzyme (superoxide dismutase and catalase) activities of the extracts were measured in hydrogen peroxide (H2O2)-stressed HepG2 cells.
RESULTS
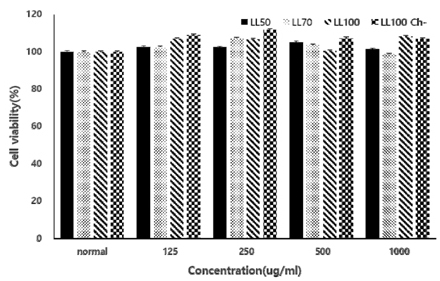
The total polyphenol content, DPPH and ABTS radical scavenging activities, and FRAP value of the extracts increased in an ethanol concentration-dependent manner. The antioxidant activities of the chlorophyll-removal extracts were much higher than those of the chlorophyll-containing extracts. Cytotoxicity was not observed in HepG2 cells with extracts up to 1,000 µg/mL. All extracts inhibited ROS production in a concentration-dependent manner from 31.3 µg/mL and inhibited DNA damage at 250 µg/mL. The SOD and catalase activities of cell lines treated with the extracts and H2O2 were similar to those of normal cells, indicating a strong protective effect.
CONCLUSION
Lycium barbarum leaf extracts had high antioxidant activities and protected H2O2-stressed HepG2 cells. Since the chlorophyll-removal extract exhibited higher antioxidant activities than the chlorophyll-containing ones and the cytoprotective effect was similar, chlorophyll removal extract of Lycium barbarum leaf could be developed as ingredients of functional food and cosmetics.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Anti-inflammatory effects of fruit and leaf extracts of
Lycium barbarum in lipopolysaccharide-stimulated RAW264.7 cells and animal model∗
Su-Mi Bae, Ji-Eun Kim, Eun-Young Bae, Kyung-Ah Kim, Sun Yung Ly
J Nutr Health. 2019;52(2):129-138. doi: 10.4163/jnh.2019.52.2.129.
Reference
-
1. Lee BC, Park JS, Kwak TS, Moon CS. Variation of chemical properties in collected boxthorn varieties. Korean J Breed. 1998; 30(3):267–272.2. Kwon KD, Park WJ, Kim SA. Buy decision making factors and marketing strategies of Lycium chinense: focused on Cheongyang region. Korean J Agric Manage Policy. 2007; 34(2):422–443.3. Park YJ, Kim M, Bae SJ. Enhancement of anticarcinogenic effect by combination of Lycii fructus with vitamin C. J Korean Soc Food Sci Nutr. 2002; 31(1):143–148.4. Park JS, Park JD, Lee BC, Choi KJ, Ra SW, Chang KW. Effects of extracts from various parts of Lycium chinense Mill. on proliferation of mouse spleen cells. Korean J Med Crop Sci. 2000; 8(4):291–296.5. Kang K, Jung J, Koh KH, Lee CH. Hepatoprotective effects of Lycium chinense mill fruit extracts and fresh fruit juice. Korean J Food Sci Technol. 2006; 38(1):99–103.6. Sung SH, Park SH. Effect of Lycii Fructus powder on lipid metabolism in 1% cholesterol fed rats. Korean J Food Cult. 2008; 23(4):521–528.7. Kim TS, Park WJ, Ko SB, Kang MH. Development of extracts of Lycii folium having high antioxidant activity. J Korean Soc Food Sci Nutr. 2008; 37(10):1318–1322.
Article8. Chernomorsky SA, Segelman AB. Biological activities of chlorophyll derivatives. N J Med. 1988; 85(8):669–673.9. Solymosi K, Mysliwa-Kurdziel B. Chlorophylls and their derivatives used in food industry and medicine. Mini Rev Med Chem. 2017; 17(13):1194–1222.
Article10. Skovsen E, Snyder JW, Lambert JD, Ogilby PR. Lifetime and diffusion of singlet oxygen in a cell. J Phys Chem B. 2005; 109(18):8570–8573.
Article11. Nurhayati N, Suendo V. Isolation of chlorophyll a from spinach leaves and modification of center ion with Zn2+: study on its optical stability. Matematika Sains. 2011; 16(2):65–70.12. Özkan G, Ersus Bilek S. Enzyme-assisted extraction of stabilized chlorophyll from spinach. Food Chem. 2015; 176:152–157.
Article13. Tanielian C, Wolff C. Mechanism of physical quenching of singlet molecular oxygen by chlorophylls and related compounds of biological interest. Photochem Photobiol. 1988; 48(3):277–280.
Article14. Sánchez-Valle V, Chávez-Tapia NC, Uribe M, Méndez-Sánchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem. 2012; 19(28):4850–4860.15. Feng Y, Wang N, Ye X, Li H, Feng Y, Cheung F, et al. Hepatoprotective effect and its possible mechanism of Coptidis rhizoma aqueous extract on carbon tetrachloride-induced chronic liver hepatotoxicity in rats. J Ethnopharmacol. 2011; 138(3):683–690.
Article16. Singal AK, Jampana SC, Weinman SA. Antioxidants as therapeutic agents for liver disease. Liver Int. 2011; 31(10):1432–1448.
Article17. Palma HE, Wolkmer P, Gallio M, Corrêa MM, Schmatz R, Thomé GR, et al. Oxidative stress parameters in blood, liver and kidney of diabetic rats treated with curcumin and/or insulin. Mol Cell Biochem. 2014; 386(1-2):199–210.
Article18. Lee CK, Kim NY, Han YN, Choi JW. Effects of pretreated Korean red ginseng on carbon tetrachloride and galactosamine-induced hepatotoxicity in rats. J Ginseng Res. 2003; 27(1):1–10.19. Folin O, Denis W. On phosphotungstic-phosphomolybdic compounds as color reagents. J Biol Chem. 1912; 12:239–243.
Article20. Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958; 181(4617):1199–1200.
Article21. Fellegrini N, Ke R, Yang M, Rice-Evans C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2′-azinobis (3-ethylenebenzothiazoline-6-sulfonic acid radical cation decolorization assay. Methods Enzymol. 1999; 299:379–389.22. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996; 239(1):70–76.
Article23. Skotti E, Anastasaki E, Kanellou G, Polissiou M, Tarantilis PA. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind Crops Prod. 2014; 53:46–54.
Article24. Liu SC, Lin JT, Hu CC, Shen BY, Chen TY, Chang YL, et al. Phenolic compositions and antioxidant attributes of leaves and stems from three inbred varieties of Lycium chinense Miller harvested at various times. Food Chem. 2017; 215:284–291.
Article25. Nirmal NP, Benjakul S. Use of tea extracts for inhibition of polyphenoloxidase and retardation of quality loss of Pacific white shrimp during iced storage. Lebenson Wiss Technol. 2011; 44(4):924–932.
Article26. Rattaya S, Benjakul S, Prodpran T. Extraction, antioxidative, and antimicrobial activities of brown seaweed extracts, Turbinaria ornata and Sargassum polycystum, grown in Thailand. Int Aquat Res. 2015; 7(1):1–16.
Article27. Olatunde OO, Benjakul S, Vongkamjan K. Antioxidant and antibacterial properties of guava leaf extracts as affected by solvents used for prior dechlorophyllization. J Food Biochem. 2018; 42(5):e12600.
Article28. Benjakul S, Kittiphattanabawon P, Shahidi F, Maqsood S. Antioxidant activity and inhibitory effects of lead(Leucaena leucocephala) seed extracts against lipid oxidation in model systems. Food Sci Technol Int. 2013; 19(4):365–376.29. Khalaf NA, Shakya AK, Al-Othman A, El-Agbar Z, Farah H. Antioxidant activity of some common plants. Turk J Biol. 2008; 32:51–55.30. Swargiary A, Daimari A, Daimari M, Basumatary N, Narzary E. Phytochemicals, antioxidant, and anthelmintic activity of selected traditional wild edible plants of lower Assam. Indian J Pharmacol. 2016; 48(4):418–423.
Article31. Benjakul S, Kittiphattanabawon P, Sumpavapol P, Maqsood S. Antioxidant activities of lead (Leucaena leucocephala) seed as affected by extraction solvent, prior dechlorophyllisation and drying methods. J Food Sci Technol. 2014; 51(11):3026–3037.
Article32. Lanfer-Marquez UM, Barros RM, Sinnecker P. Antioxidant activity of chlorophylls and their derivatives. Food. 2005; 38(8-9):885–891.
Article33. Sánchez-González I, Jiménez-Escrig A, Saura-Calixto F. In vitro antioxidant activity of coffees brewed using different procedures (Italian, espresso and filter). Food Chem. 2005; 90(1-2):133–139.
Article34. Albishi T, John IA, Al-Khalifa AS, Shahidi F. Antioxidative phenolic constituents of skins of onion varieties and their activities. J Funct Foods. 2013; 5(3):1191–1203.
Article35. Yoo HJ, Ahn C, Narantuya L. Extractions of chlorophyll from spinach and mate powders and their dyeability on fabrics. J Korean Soc Clothing Text. 2013; 37(3):413–423.
Article36. Conforti F, Ioele G, Statti GA, Marrelli M, Ragno G, Menichini F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem Toxicol. 2008; 46(10):3325–3332.
Article37. Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G, et al. Lycium barbarum polysaccharides protect human lens epithelial cells against oxidative stress-induced apoptosis and senescence. PLoS One. 2014; 9(10):e110275.
Article38. Ceccarini MR, Vannini S, Cataldi S, Moretti M, Villarini M, Fioretti B, Codini M, et al. In vitro protective effects of Lycium barbarum berries cultivated in Umbria(Italy) on human hepatocellular carcinoma cells. BioMed Res Int. 2016; 2016:7529521.39. Bobek P. Dietary tomato and grape pomace in rats: effect on lipids in serum and liver, and on antioxidant status. Br J Biomed Sci. 1999; 56(2):109–113.40. Erzurum SC, Lemarchand P, Rosenfeld MA, Yoo JH, Crystal RG. Protection of human endothelial cells from oxidant injury by adenovirus-mediated transfer of the human catalase cDNA. Nucleic Acids Res. 1993; 21(7):1607–1612.
Article41. Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970; 34(1):30–38.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effects of fruit and leaf extracts of Lycium barbarum in lipopolysaccharide-stimulated RAW264.7 cells and animal model
- Protective effect of chlorophyllremoved ethanol extract of Lycium barbarum leaves against nonalcoholic fatty liver disease
- Protective effect of Lycium barbarum leaf extracts on atopic dermatitis: in vitro and in vivo studies
- Antioxidant activity and analysis of proanthocyanidins from pine (Pinus densiflora) needles
- Antioxidant Activity of Vitex rotundifolia Seeds and Phytochemical Analysis Using HPLC-PDA