J Nutr Health.
2019 Dec;52(6):529-539. 10.4163/jnh.2019.52.6.529.
Antioxidant and antiobesity activities of oral treatment with ethanol extract from sprout of evening primrose (Oenothera laciniata) in high fat diet-induced obese mice
- Affiliations
-
- 1Institute on Aging, Seoul National University, Seoul 03080, Korea. kwakcs@snu.ac.kr
- 2Cell2in, Inc., Seoul 03080, Korea.
- 3Dain Natural Co., Ltd. Seoul 04788, Korea.
- 4Department of Biochemistry and Molecular Biology, Seoul National University College of Medicine, Seoul 03080, Korea.
- KMID: 2466667
- DOI: http://doi.org/10.4163/jnh.2019.52.6.529
Abstract
- PURPOSE
Sprouts of evening primrose (Oenothera laciniata, OL) were reported to have high contents of flavonoids and potent antioxidant activity. This study examined the antioxidant and antiobesity activities of OL sprouts to determine if they could be a natural health-beneficial resource preventing obesity and oxidative stress.
METHODS
OL sprouts were extracted with 50% ethanol, evaporated, and lyophilized (OLE). The in vitro antioxidant activity of OLE was examined using four different tests. The antiobesity activity and in vivo antioxidant activity from OLE consumption were examined using high fat diet-induced obese (DIO) C57BL/6 mice.
RESULTS
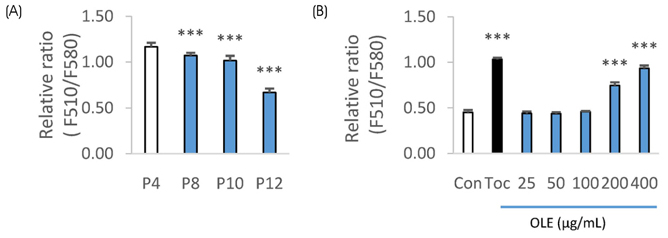
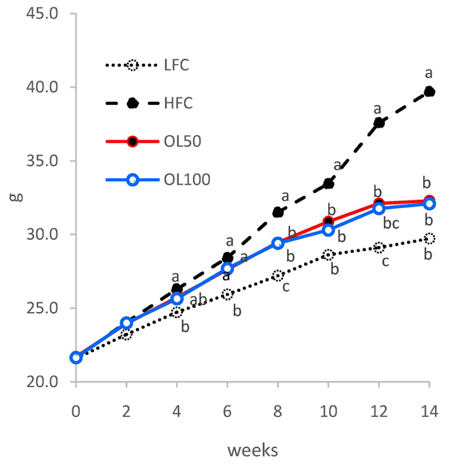
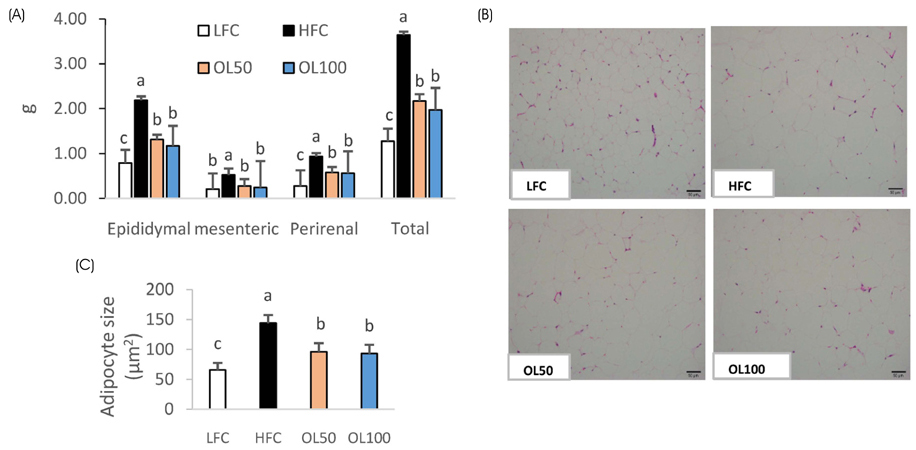
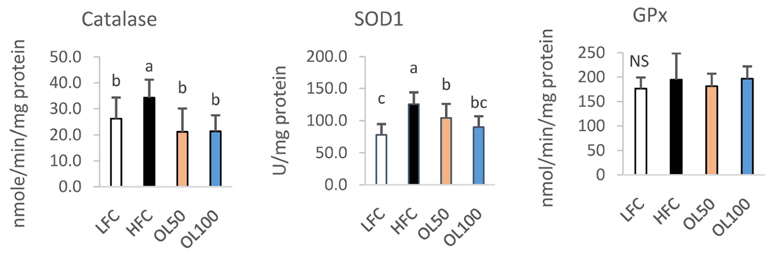
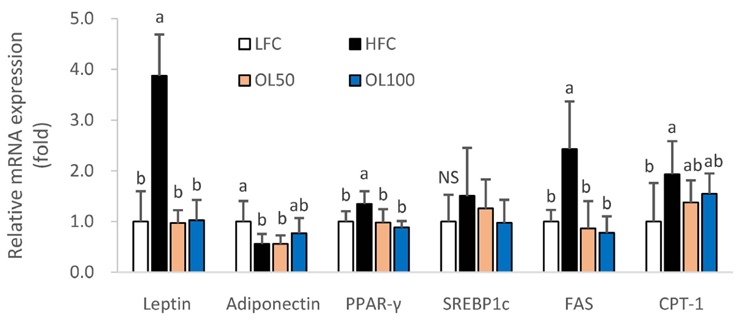
The ICâ‚…â‚€ for the 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging and superoxide dismutase (SOD)-like activities of OLE were 26.2 µg/mL and 327.6 µg/mL, respectively. OLE exhibited the ferric reducing antioxidant power (FRAP) activity of 56.7 µg ascorbic acid eq./mL at 100 µg/mL, and an increased glutathione level by 65.1% at 200 µg/mL compared to the control in the hUC-MSC stem cells. In an animal study, oral treatment with 50 mg or 100 mg of OLE/kg body weight for 14 weeks reduced the body weight gain, visceral fat content, fat cell size, blood leptin, and triglyceride levels, as well as the atherogenic index compared to the high fat diet control group (HFC) (p < 0.05). The blood malondialdehyde (MDA) level and the catalase and SOD-1 activities in adipose tissue were reduced significantly by the OLE treatment compared to HFC as well (p < 0.05). In epididymal adipose tissue, the OLE treatment reduced the mRNA expression of leptin, PPAR-γ and FAS significantly (p < 0.05) compared to HFC while it increased adiponectin expression (p < 0.05).
CONCLUSION
OLE consumption has potent antioxidant and antiobesity activities via the suppression of oxidative stress and lipogenesis in DIO mice. Therefore, OLE could be a good candidate as a natural resource to develop functional food products that prevent obesity and oxidative stress.
MeSH Terms
-
Adipocytes
Adipokines
Adiponectin
Adipose Tissue
Animals
Ascorbic Acid
Body Weight
Catalase
Diet, High-Fat
Ethanol*
Flavonoids
Functional Food
Glutathione
In Vitro Techniques
Intra-Abdominal Fat
Leptin
Lipogenesis
Malondialdehyde
Mice
Mice, Obese*
Natural Resources
Obesity
Oenothera biennis*
Oxidative Stress
RNA, Messenger
Stem Cells
Superoxide Dismutase
Triglycerides
Adipokines
Adiponectin
Ascorbic Acid
Catalase
Ethanol
Flavonoids
Glutathione
Leptin
Malondialdehyde
RNA, Messenger
Superoxide Dismutase
Figure
Reference
-
1. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114(12):1752–1761.
Article2. Nam YR, Won SB, Chung YS, Kwak CS, Kwon YH. Inhibitory effects of Doenjang, Korean traditional fermented soybean paste, on oxidative stress and inflammation in adipose tissue of mice fed a high-fat diet. Nutr Res Pract. 2015; 9(3):235–241.3. da Costa GF, Santos IB, de Bem GF, Cordeiro VS, da Costa CA, de Carvalho LC, et al. The beneficial effect of anthocyanidin-rich Vitis vinifera L. grape skin extract on metabolic changes induced by high-fat diet in mice involves antiinflammatory and antioxidant actions. Phytother Res. 2017; 31(10):1621–1632.4. Charradi K, Elkahoui S, Limam F, Aouani E. High-fat diet induced an oxidative stress in white adipose tissue and disturbed plasma transition metals in rat: prevention by grape seed and skin extract. J Physiol Sci. 2013; 63(6):445–455.
Article5. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011; 29:415–445.
Article6. Galinier A, Carrière A, Fernandez Y, Carpéné C, André M, Caspar-Bauguil S, et al. Adipose tissue proadipogenic redox changes in obesity. J Biol Chem. 2006; 281(18):12682–12687.
Article7. Carrasco-Pozo C, Cires MJ, Gotteland M. Quercetin and epigallocatechin gallate in the prevention and treatment of obesity: from molecular to clinical studies. J Med Food. 2019; 22(8):753–770.
Article8. Kowalska K, Olejnik A, Szwajgier D, Olkowicz M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS One. 2017; 12(11):e0188583.
Article9. Lee OH, Seo MJ, Choi HS, Lee BY. Pycnogenol® inhibits lipid accumulation in 3T3-L1 adipocytes with the modulation of reactive oxygen species (ROS) production associated with antioxidant enzyme responses. Phytother Res. 2012; 26(3):403–411.
Article10. Zhao Y, Chen B, Shen J, Wan L, Zhu Y, Yi T, et al. The beneficial effects of quercetin, curcumin, and resveratrol in obesity. Oxid Med Cell Longev. 2017; 2017:1459497.
Article11. Kim HJ, Kim B, Mun EG, Jeong SY, Cha YS. The antioxidant activity of steamed ginger and its protective effects on obesity induced by high-fat diet in C57BL/6J mice. Nutr Res Pract. 2018; 12(6):503–511.
Article12. Kim JH, Kim OK, Yoon HG, Park J, You Y, Kim K, et al. Anti-obesity effect of extract from fermented Curcuma longa L. through regulation of adipogenesis and lipolysis pathway in high-fat diet-induced obese rats. Food Nutr Res. 2016; 60(1):30428.13. Tuzcu Z, Orhan C, Sahin N, Juturu V, Sahin K. Cinnamon polyphenol extract inhibits hyperlipidemia and inflammation by modulation of transcription factors in high-fat diet-fed rats. Oxid Med Cell Longev. 2017; 2017:1583098.
Article14. Castro-Barquero S, Lamuela-Raventós RM, Doménech M, Estruch R. Relationship between Mediterranean dietary polyphenol intake and obesity. Nutrients. 2018; 10(10):1523.
Article15. Naowaboot J, Wannasiri S, Pannangpetch P. Morin attenuates hepatic insulin resistance in high-fat-diet-induced obese mice. J Physiol Biochem. 2016; 72(2):269–280.
Article16. Fenni S, Hammou H, Astier J, Bonnet L, Karkeni E, Couturier C, et al. Lycopene and tomato powder supplementation similarly inhibit high-fat diet induced obesity, inflammatory response, and associated metabolic disorders. Mol Nutr Food Res. 2017; 61(9):1601083.
Article17. González-Castejón M, Rodriguez-Casado A. Dietary phytochemicals and their potential effects on obesity: a review. Pharmacol Res. 2011; 64(5):438–455.
Article18. Timoszuk M, Bielawska K, Skrzydlewska E. Evening primrose (Oenothera biennis) biological activity dependent on chemical composition. Antioxidants. 2018; 7(8):108.
Article19. Yoon WJ, Ham YM, Yoo BS, Moon JY, Koh J, Hyun CG. Oenothera laciniata inhibits lipopolysaccharide induced production of nitric oxide, prostaglandin E2, and proinflammatory cytokines in RAW264.7 macrophages. J Biosci Bioeng. 2009; 107(4):429–438.
Article20. Garnica S, Czerwinska ME, Piwowarski JP, Ziaja M, Kiss AK. Chemical composition, antioxidative and anti-inflammatory activity of extracts prepared from aerial parts of Oenothera biennis L. anf Oenothera paradoxa Hudziok obtained after seeds cultivation. J Agric Food Chem. 2013; 61(4):801–810.21. Kwak CS, Lee JH. In vitro antioxidant and antiinflammatory effects of ethanol extracts from sprout of evening primrose (Oenothera laciniata) and gooseberry (Actinidia arguta). J Korean Soc Food Sci Nutr. 2014; 43(2):207–215.
Article22. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974; 47(3):469–474.
Article23. Jeong EM, Shin JW, Lim J, Kim JH, Kang H, Yin Y, et al. Monitoring glutathione dynamics and heterogeneity in living stem cells. Int J Stem Cells. 2019; 12(2):367–379.
Article24. Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105:121–126.25. Tappel AL. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978; 52:506–513.26. Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995; 43(1):27–32.
Article27. Yang SL, Yu PL, Chung KR. The glutathione peroxidase-mediated reactive oxygen species resistance, fungicide sensitivity and cell wall construction in the citrus fungal pathogen Alternaria alternata. Environ Microbiol. 2016; 18(3):923–935.28. Lee YS, Kim AY, Choi JW, Kim M, Yasue S, Son HJ, et al. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol Endocrinol. 2008; 22(9):2176–2189.
Article29. Kobayashi H, Matsuda M, Fukuhara A, Komuro R, Shimomura I. Dysregulated glutathione metabolism links to impaired insulin action in adipocytes. Am J Physiol Endocrinol Metab. 2009; 296(6):E1326–E1334.
Article30. Wu T, Yin J, Zhang G, Long H, Zheng X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol Nutr Food Res. 2016; 60(3):687–694.
Article31. Mounien L, Tourniaire F, Landrier JF. Anti-obesity effect of carotenoids: direct impact on adipose tissue and adipose tissue-driven indirect effects. Nutrients. 2019; 11(7):1562.
Article32. Rivera L, Morón R, Sanchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity (Silver Spring). 2008; 16(9):2081–2087.
Article33. Dong J, Zhang X, Zhang L, Bian HX, Xu N, Bao B, et al. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: a mechanism including AMPKα1/SIRT1. J Lipid Res. 2014; 55(3):363–374.
Article34. Cialdella-Kam L, Ghosh S, Meaney MP, Knab AM, Shanely RA, Nieman DC. Quercetin and green tea extract supplementation downregulates genes related to tissue inflammatory responses to a 12-week high fat-diet in mice. Nutrients. 2017; 9(7):E773.
Article35. Lee JS, Cha YJ, Lee KH, Yim JE. Onion peel extract reduces the percentage of body fat in overweight and obese subjects: a 12-week, randomized, double-blind, placebo-controlled study. Nutr Res Pract. 2016; 10(2):175–181.
Article36. Gentile D, Fornai M, Pellegrini C, Colucci R, Benvenuti L, Duranti E, et al. Luteolin prevents cardiometabolic alterations and vascular dysfunction in mice with HFD-induced obesity. Front Pharmacol. 2018; 9:1094.
Article37. Liu Y, Fu X, Lan N, Li S, Zhang J, Wang S, et al. Luteolin protects against high fat diet-induced cognitive deficits in obesity mice. Behav Brain Res. 2014; 267:178–188.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Antioxidant and antiobesity activities of oral treatment with ethanol extract from sprout of evening primrose (Oenothera laciniata) in high fat diet-induced obese mice
- Evening Primrose (Oenothera biennis) Oil in Management of Female Ailments
- Effects of Acorn Supplementation on Lipid Profiles and Antioxidant Enzyme Activities in High Fat Diet-Induced Obese Rats
- Effect of Eveing Primrose on Plasma Cholesterol Levels and Immune Response to Sheep Erythrocytes
- Dual effects of a mixture of grape pomace (Campbell Early) and Omija fruit ethanol extracts on lipid metabolism and the antioxidant defense system in diet-induced obese mice