Yonsei Med J.
2016 Jan;57(1):247-253. 10.3349/ymj.2016.57.1.247.
Galpha12 Protects Vascular Endothelial Cells from Serum Withdrawal-Induced Apoptosis through Regulation of miR-155
- Affiliations
-
- 1Department of Endocrinology, Brain Korea 21 Plus Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. ejlee423@yuhs.ac
- 2Severance Hospital Integrative Research Institute for Cerebral & Cardiovascular Disease, Yonsei University College of Medicine, Seoul, Korea. seo99@yuhs.ac
- KMID: 2466377
- DOI: http://doi.org/10.3349/ymj.2016.57.1.247
Abstract
- PURPOSE
Apoptosis of vascular endothelial cells is a type of endothelial damage that is associated with the pathogenesis of cardiovascular diseases such as atherosclerosis. Heterotrimeric GTP-binding proteins (G proteins), including the alpha 12 subunit of G protein (Galpha12), have been found to modulate cellular proliferation, differentiation, and apoptosis of numerous cell types. However, the role of Galpha12 in the regulation of apoptosis of vascular cells has not been elucidated. We investigated the role of Galpha12 in serum withdrawal-induced apoptosis of human umbilical vein endothelial cells (HUVECs) and its underlying mechanisms.
MATERIALS AND METHODS
HUVECs were transfected with Galpha12 small-interfering RNA (siRNA) to knockdown the endogenous Galpha12 expression and were serum-deprived for 6 h to induce apoptosis. The apoptosis of HUVECs were assessed by Western blotting and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. The expressions of microRNAs were analyzed by quantitative real-time PCR.
RESULTS
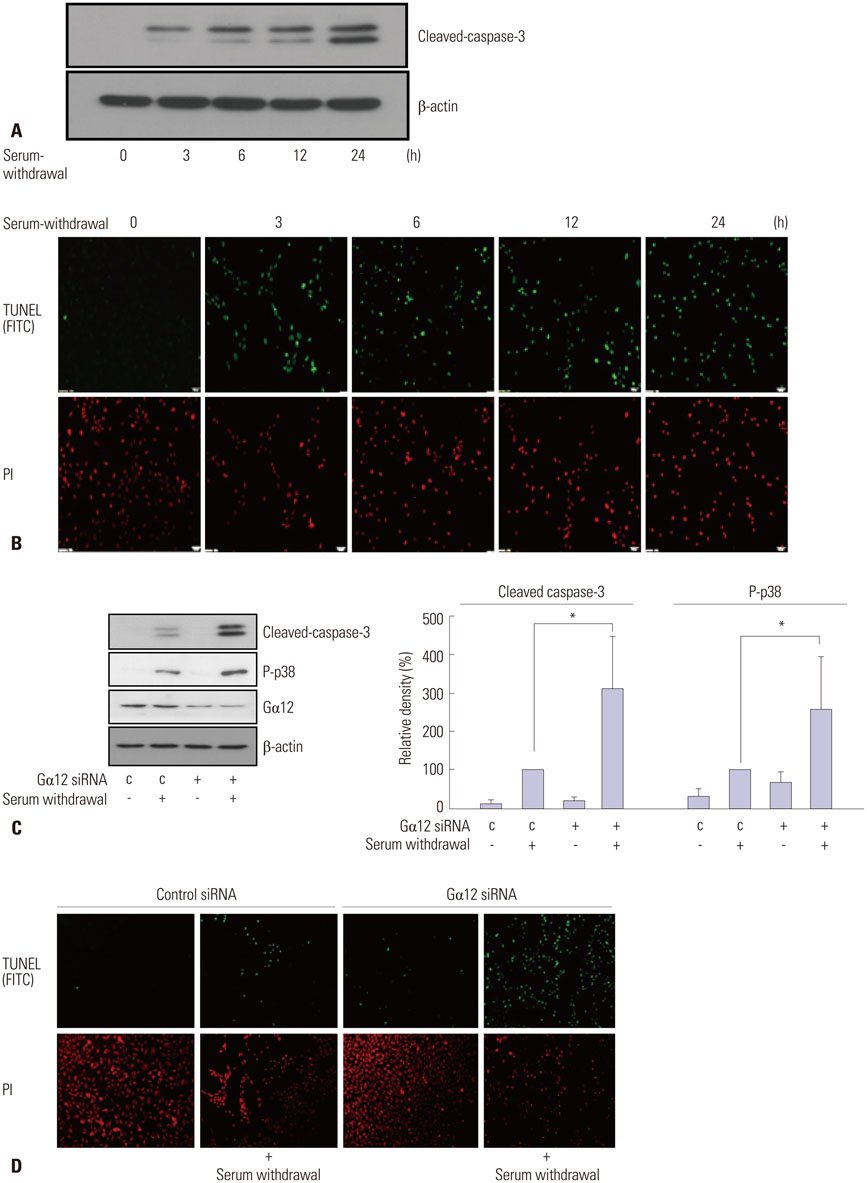
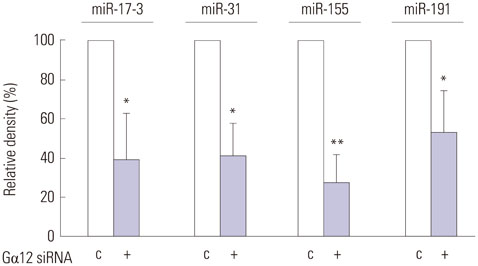
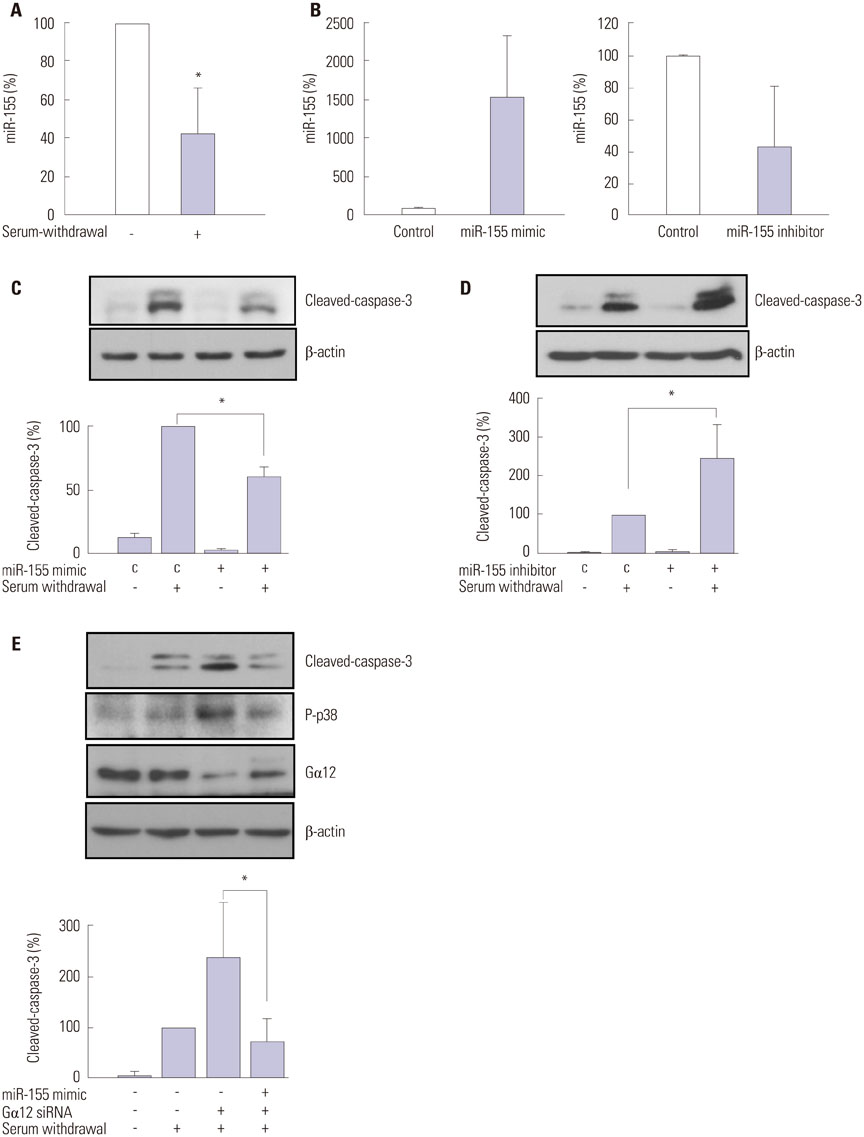
Knockdown of Galpha12 with siRNA augmented the serum withdrawal-induced apoptosis of HUVECs and markedly repressed the expression of microRNA-155 (miR-155). Serum withdrawal-induced apoptosis of HUVECs was inhibited by the overexpression of miR-155 and increased significantly due to the inhibition of miR-155. Notably, the elevation of miR-155 expression prevented increased apoptosis of Galpha12-deficient HUVECs.
CONCLUSION
From these results, we conclude that Galpha12 protects HUVECs from serum withdrawal-induced apoptosis by retaining miR-155 expression. This suggests that Galpha12 might play a protective role in vascular endothelial cells by regulating the expression of microRNAs.
Keyword
MeSH Terms
-
*Apoptosis
Atherosclerosis/*blood/genetics/immunology
Cell Proliferation
Endothelial Cells/*metabolism
GTP-Binding Protein alpha Subunits, G12-G13/*genetics
Gene Expression Profiling
Gene Expression Regulation
Human Umbilical Vein Endothelial Cells/cytology
Humans
MicroRNAs/*metabolism
Protective Agents
*RNA, Small Interfering
Real-Time Polymerase Chain Reaction
*Transfection
GTP-Binding Protein alpha Subunits, G12-G13
MicroRNAs
Protective Agents
RNA, Small Interfering
Figure
Reference
-
1. Hepler JR, Gilman AG. G proteins. Trends Biochem Sci. 1992; 17:383–387.
Article2. Xu N, Bradley L, Ambdukar I, Gutkind JS. A mutant alpha subunit of G12 potentiates the eicosanoid pathway and is highly oncogenic in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1993; 90:6741–6745.
Article3. Gan CP, Patel V, Mikelis CM, Zain RB, Molinolo AA, Abraham MT, et al. Heterotrimeric G-protein alpha-12 (Gα12) subunit promotes oral cancer metastasis. Oncotarget. 2014; 5:9626–9640.
Article4. Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005; 435:834–838.
Article5. Quintavalle M, Condorelli G, Elia L. Arterial remodeling and atherosclerosis: miRNAs involvement. Vascul Pharmacol. 2011; 55:106–110.
Article6. Ma X, Ma C, Zheng X. MicroRNA-155 in the pathogenesis of atherosclerosis: a conflicting role. Heart Lung Circ. 2013; 22:811–818.
Article7. Yanamadala V, Negoro H, Denker BM. Heterotrimeric G proteins and apoptosis: intersecting signaling pathways leading to context dependent phenotypes. Curr Mol Med. 2009; 9:527–545.
Article8. Seo M, Cho CH, Lee YI, Shin EY, Park D, Bae CD, et al. Cdc42-dependent mediation of UV-induced p38 activation by G protein betagamma subunits. J Biol Chem. 2004; 279:17366–17375.
Article9. Ro S, Park C, Jin J, Sanders KM, Yan W. A PCR-based method for detection and quantification of small RNAs. Biochem Biophys Res Commun. 2006; 351:756–763.
Article10. Suárez Y, Wang C, Manes TD, Pober JS. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. J Immunol. 2010; 184:21–25.
Article11. Liu T, Shen D, Xing S, Chen J, Yu Z, Wang J, et al. Attenuation of exogenous angiotensin II stress-induced damage and apoptosis in human vascular endothelial cells via microRNA-155 expression. Int J Mol Med. 2013; 31:188–196.
Article12. Goldsmith ZG, Ha JH, Jayaraman M, Dhanasekaran DN. Lysophosphatidic Acid Stimulates the Proliferation of Ovarian Cancer Cells via the gep Proto-Oncogene Gα(12). Genes Cancer. 2011; 2:563–575.
Article13. Kumar RN, Radhika V, Audigé V, Rane SG, Dhanasekaran N. Proliferation-specific genes activated by Galpha(12): a role for PDGFRalpha and JAK3 in Galpha(12)-mediated cell proliferation. Cell Biochem Biophys. 2004; 41:63–73.14. Kelly P, Stemmle LN, Madden JF, Fields TA, Daaka Y, Casey PJ. A role for the G12 family of heterotrimeric G proteins in prostate cancer invasion. J Biol Chem. 2006; 281:26483–26490.
Article15. Kelly P, Moeller BJ, Juneja J, Booden MA, Der CJ, Daaka Y, et al. The G12 family of heterotrimeric G proteins promotes breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2006; 103:8173–8178.
Article16. Kim YM, Lim SC, Han CY, Kay HY, Cho IJ, Ki SH, et al. G(alpha)12/13 induction of CYR61 in association with arteriosclerotic intimal hyperplasia: effect of sphingosine-1-phosphate. Arterioscler Thromb Vasc Biol. 2011; 31:861–869.
Article17. Yanamadala V, Negoro H, Gunaratnam L, Kong T, Denker BM. Galpha12 stimulates apoptosis in epithelial cells through JNK1-mediated Bcl-2 degradation and up-regulation of IkappaBalpha. J Biol Chem. 2007; 282:24352–24363.
Article18. Berestetskaya YV, Faure MP, Ichijo H, Voyno-Yasenetskaya TA. Regulation of apoptosis by alpha-subunits of G12 and G13 proteins via apoptosis signal-regulating kinase-1. J Biol Chem. 1998; 273:27816–27823.
Article19. Choy JC, Granville DJ, Hunt DW, McManus BM. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol. 2001; 33:1673–1690.
Article20. Xu F, Sun Y, Chen Y, Sun Y, Li R, Liu C, et al. Endothelial cell apoptosis is responsible for the formation of coronary thrombotic atherosclerotic plaques. Tohoku J Exp Med. 2009; 218:25–33.
Article21. Li S, Chen T, Zhong Z, Wang Y, Li Y, Zhao X. microRNA-155 silencing inhibits proliferation and migration and induces apoptosis by upregulating BACH1 in renal cancer cells. Mol Med Rep. 2012; 5:949–954.
Article22. Huang RS, Hu GQ, Lin B, Lin ZY, Sun CC. MicroRNA-155 silencing enhances inflammatory response and lipid uptake in oxidized low-density lipoprotein-stimulated human THP-1 macrophages. J Investig Med. 2010; 58:961–967.
Article23. Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012; 122:4190–4202.
Article24. Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011; 215:286–293.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Silibinin-Induced Apoptosis and Downregulation of MicroRNA-21 and MicroRNA-155 in MCF-7 Human Breast Cancer Cells
- Knockdown of Long Non-Coding RNA NEAT1 Inhibits Proliferation and Invasion and Induces Apoptosis of Osteosarcoma by Inhibiting miR-194 Expression
- Carbon monoxide prevents TNF-α-induced eNOS downregulation by inhibiting NF-κB-responsive miR-155-5p biogenesis
- Fluoxetine Protects against Big Endothelin-1 Induced Anti-Apoptosis by Rescuing Kv1.5 Channels in Human Pulmonary Arterial Smooth Muscle Cells
- The Anti-apoptotic Effect of Adrenomedullin on Vascular Endothelial Cells in Radiocontrast Media-induced Apoptosis