Korean J Physiol Pharmacol.
2019 Nov;23(6):483-491. 10.4196/kjpp.2019.23.6.483.
Cordycepin protects against β-amyloid and ibotenic acid-induced hippocampal CA1 pyramidal neuronal hyperactivity
- Affiliations
-
- 1School of Life Science, Jiangxi Science & Technology Normal University, Nanchang, Jiangxi 330013, PR China.
- 2School of Pharmacy, Jiangxi University of Traditional Chinese Medicine, Nanchang, Jiangxi 330004, PR China. 286529404@qq.com
- 3School of Sport Science, Jiangxi Science & Technology Normal University, Nanchang, Jiangxi 330013, PR China.
- KMID: 2461041
- DOI: http://doi.org/10.4196/kjpp.2019.23.6.483
Abstract
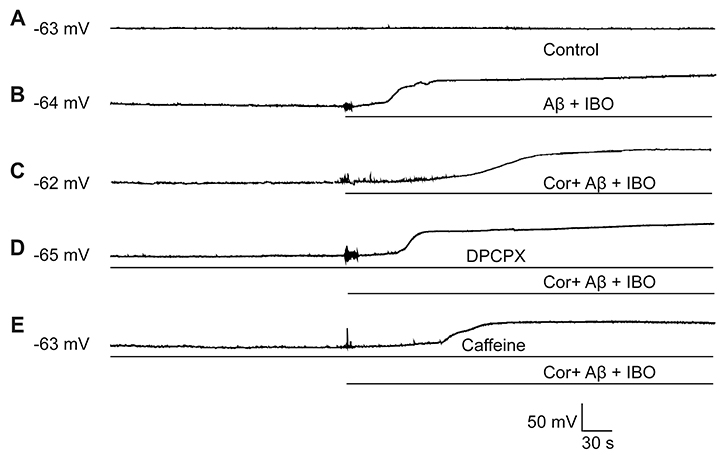
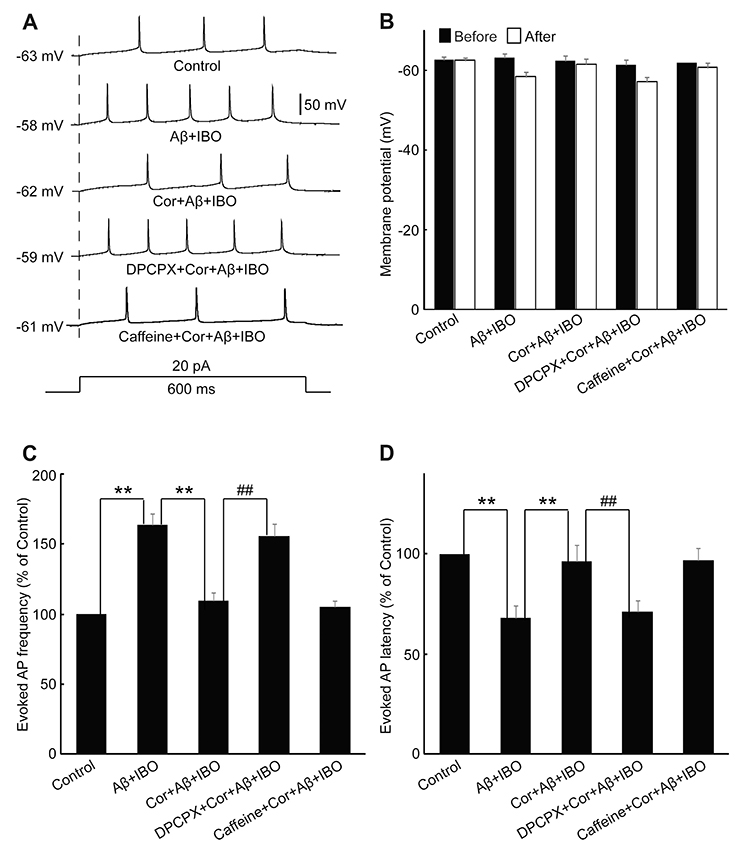
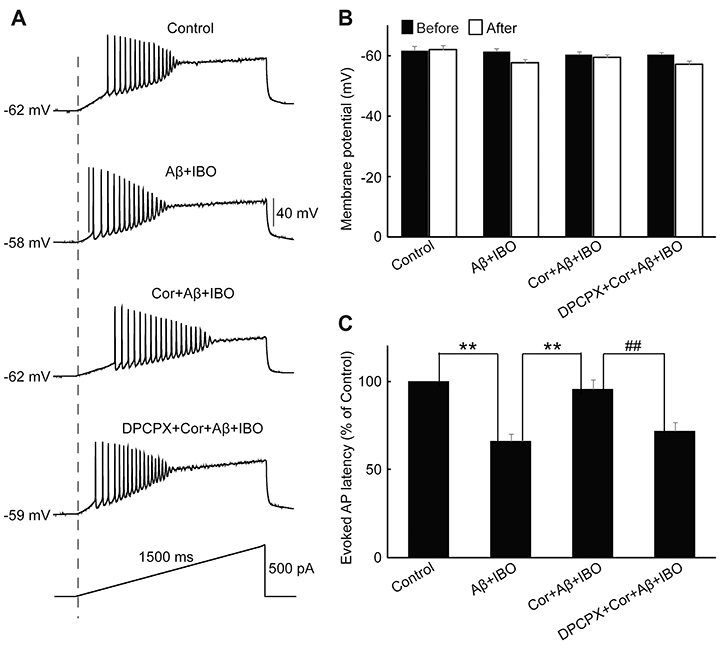
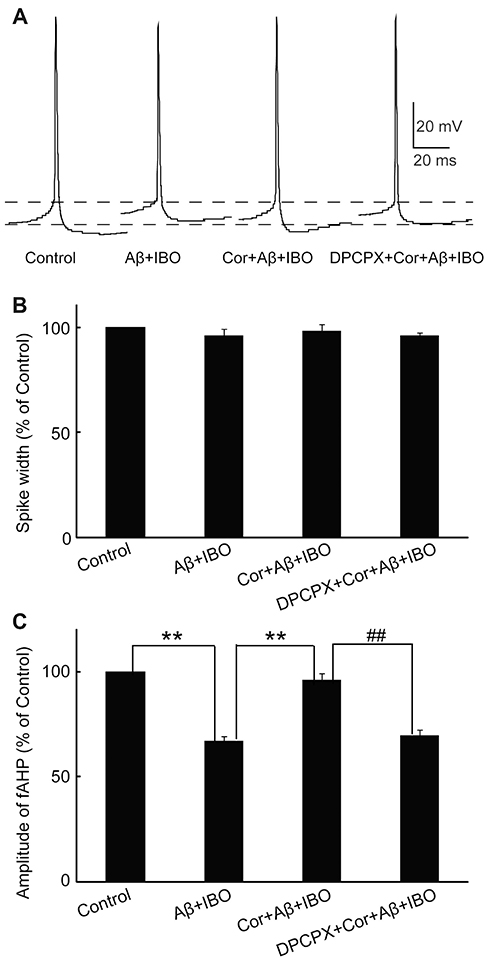
- Cordycepin exerts neuroprotective effects against excitotoxic neuronal death. However, its direct electrophysiological evidence in Alzheimer's disease (AD) remains unclear. This study aimed to explore the electrophysiological mechanisms underlying the protective effect of cordycepin against the excitotoxic neuronal insult in AD using whole-cell patch clamp techniques. β-Amyloid (Aβ) and ibotenic acid (IBO)-induced injury model in cultured hippocampal neurons was used for the purpose. The results revealed that cordycepin significantly delayed Aβ+ IBO-induced excessive neuronal membrane depolarization. It increased the onset time/latency, extended the duration, and reduced the slope in both slow and rapid depolarization. Additionally, cordycepin reversed the neuronal hyperactivity in Aβ+ IBO-induced evoked action potential (AP) firing, including increase in repetitive firing frequency, shortening of evoked AP latency, decrease in the amplitude of fast afterhyperpolarization, and increase in membrane depolarization. Further, the suppressive effect of cordycepin against Aβ+ IBO-induced excessive neuronal membrane depolarization and neuronal hyperactivity was blocked by DPCPX (8-cyclopentyl-1,3-dipropylxanthine, an adenosine Aâ‚ receptor-specific blocker). Collectively, these results revealed the suppressive effect of cordycepin against the Aβ+ IBO-induced excitotoxic neuronal insult by attenuating excessive neuronal activity and membrane depolarization, and the mechanism through the activation of Aâ‚R is strongly recommended, thus highlighting the therapeutic potential of cordycepin in AD.
MeSH Terms
Figure
Reference
-
1. Liu X, Hou D, Lin F, Luo J, Xie J, Wang Y, Tian Y. The role of neurovascular unit damage in the occurrence and development of Alzheimer's disease. Rev Neurosci. 2019; 30:477–484.
Article2. Alzheimer's Association. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016; 12:459–509.3. Citron M. Alzheimer's disease: strategies for disease modification. Nat Rev Drug Discov. 2010; 9:387–398.
Article4. Calì T, Ottolini D, Brini M. Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson's disease. Biofactors. 2011; 37:228–240.
Article5. Jin ML, Park SY, Kim YH, Oh JI, Lee SJ, Park G. The neuroprotective effects of cordycepin inhibit glutamate-induced oxidative and ER stress-associated apoptosis in hippocampal HT22 cells. Neurotoxicology. 2014; 41:102–111.
Article6. Wang Y, Qin ZH. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 2010; 15:1382–1402.
Article7. Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009; 30:379–387.
Article8. Šišková Z, Justus D, Kaneko H, Friedrichs D, Henneberg N, Beutel T, Pitsch J, Schoch S, Becker A, von der Kammer H, Remy S. Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of Alzheimer's disease. Neuron. 2014; 84:1023–1033.
Article9. Yin H, Wang H, Zhang H, Gao N, Zhang T, Yang Z. Resveratrol attenuates Aβ-induced early hippocampal neuron excitability impairment via recovery of function of potassium channels. Neurotox Res. 2017; 32:311–324.
Article10. Zhang J, Li P, Wang Y, Liu J, Zhang Z, Cheng W, Wang Y. Ameliorative effects of a combination of baicalin, jasminoidin and cholic acid on ibotenic acid-induced dementia model in rats. PLoS One. 2013; 8:e56658.
Article11. Alberdi E, Sánchez-Gómez MV, Cavaliere F, Pérez-Samartín A, Zugaza JL, Trullas R, Domercq M, Matute C. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010; 47:264–272.12. Bieschke J, Herbst M, Wiglenda T, Friedrich RP, Boeddrich A, Schiele F, Kleckers D, Lopez del Amo JM, Grüning BA, Wang Q, Schmidt MR, Lurz R, Anwyl R, Schnoegl S, Fändrich M, Frank RF, Reif B, Günther S, Walsh DM, Wanker EE. Small-molecule conversion of toxic oligomers to nontoxic β-sheet-rich amyloid fibrils. Nat Chem Biol. 2011; 8:93–101.
Article13. Hruska Z, Dohanich GP. The effects of chronic estradiol treatment on working memory deficits induced by combined infusion of beta-amyloid (1–42) and ibotenic acid. Horm Behav. 2007; 52:297–306.14. Choi YH, Kim GY, Lee HH. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways. Drug Des Devel Ther. 2014; 8:1941–1953.15. Ramesh T, Yoo SK, Kim SW, Hwang SY, Sohn SH, Kim IW, Kim SK. Cordycepin (3′-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp Gerontol. 2012; 47:979–987.
Article16. Chaicharoenaudomrung N, Jaroonwitchawan T, Noisa P. Cordycepin induces apoptotic cell death of human brain cancer through the modulation of autophagy. Toxicol In Vitro. 2018; 46:113–121.
Article17. Olatunji OJ, Feng Y, Olatunji OO, Tang J, Ouyang Z, Su Z. Cordycepin protects PC12 cells against 6-hydroxydopamine induced neurotoxicity via its antioxidant properties. Biomed Pharmacother. 2016; 81:7–14.
Article18. Peng J, Wang P, Ge H, Qu X, Jin X. Effects of cordycepin on the microglia-overactivation-induced impairments of growth and development of hippocampal cultured neurons. PLoS One. 2015; 10:e0125902.
Article19. Cheng Z, He W, Zhou X, Lv Q, Xu X, Yang S, Zhao C, Guo L. Cordycepin protects against cerebral ischemia/reperfusion injury in vivo and in vitro. Eur J Pharmacol. 2011; 664:20–28.
Article20. Chen C, Liu XP, Jiang W, Zeng B, Meng W, Huang LP, Li YP, Sun W, Yuan CH, Yao LH. Anti-effects of cordycepin to hypoxia-induced membrane depolarization on hippocampal CA1 pyramidal neuron. Eur J Pharmacol. 2017; 796:1–6.
Article21. Lauro C, Cipriani R, Catalano M, Trettel F, Chece G, Brusadin V, Antonilli L, van Rooijen N, Eusebi F, Fredholm BB, Limatola C. Adenosine A1 receptors and microglial cells mediate CX3CL1-induced protection of hippocampal neurons against Glu-induced death. Neuropsychopharmacology. 2010; 35:1550–1559.
Article22. Song H, Huang LP, Li Y, Liu C, Wang S, Meng W, Wei S, Liu XP, Gong Y, Yao LH. Neuroprotective effects of cordycepin inhibit Aβ-induced apoptosis in hippocampal neurons. Neurotoxicology. 2018; 68:73–80.
Article23. Ford L, Crossley M, Williams T, Thorpe JR, Serpell LC, Kemenes G. Effects of Aβ exposure on long-term associative memory and its neuronal mechanisms in a defined neuronal network. Sci Rep. 2015; 5:10614.
Article24. Shen H, Pan J, Pan L, Zhang N. TRPC6 inhibited NMDA current in cultured hippocampal neurons. Neuromolecular Med. 2013; 15:389–395.
Article25. Yao LH, Li CH, Yan WW, Huang JN, Liu WX, Xiao P. Cordycepin decreases activity of hippocampal CA1 pyramidal neuron through membrane hyperpolarization. Neurosci Lett. 2011; 503:256–260.
Article26. Matthews EA, Weible AP, Shah S, Disterhoft JF. The BK-mediated fAHP is modulated by learning a hippocampus-dependent task. Proc Natl Acad Sci U S A. 2008; 105:15154–15159.
Article27. Haberman RP, Koh MT, Gallagher M. Heightened cortical excitability in aged rodents with memory impairment. Neurobiol Aging. 2017; 54:144–151.
Article28. Thomé A, Gray DT, Erickson CA, Lipa P, Barnes CA. Memory impairment in aged primates is associated with region-specific network dysfunction. Mol Psychiatry. 2016; 21:1257–1262.
Article29. Koh MT, Rosenzweig-Lipson S, Gallagher M. Selective GABA(A) α5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology. 2013; 64:145–152.
Article30. Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010; 35:1016–1025.
Article31. Yuan P, Grutzendler J. Attenuation of β-Amyloid deposition and neurotoxicity by chemogenetic modulation of neural activity. J Neurosci. 2016; 36:632–641.
Article32. Zhang D, Xiong W, Chu S, Sun C, Albensi BC, Parkinson FE. Inhibition of hippocampal synaptic activity by ATP, hypoxia or oxygen-glucose deprivation does not require CD73. PLoS One. 2012; 7:e39772.
Article33. Masino SA, Diao L, Illes P, Zahniser NR, Larson GA, Johansson B, Fredholm BB, Dunwiddie TV. Modulation of hippocampal glutamatergic transmission by ATP is dependent on adenosine a(1) receptors. J Pharmacol Exp Ther. 2002; 303:356–363.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A TUNEL and Electron Microscopic Study on the Delayed Neuronal Death of Rat CA1 Pyramidal Neurons after MCAO
- Cyanidin-3-glucoside inhibits amyloid β₂₅₋₃₅-induced neuronal cell death in cultured rat hippocampal neurons
- Temporal changes in mammalian target of rapamycin (mTOR) and phosphorylated-mTOR expressions in the hippocampal CA1 region of rat with vascular dementia
- Neurotoxic Effect of beta-Amyloid Peptide in Hippocampal Slice Culture
- Immunohistochemical Study on GTP-binding Rab6 Expression in the Hippocampal Cortices of the Alzheimer Brain