Korean J Physiol Pharmacol.
2018 Nov;22(6):689-696. 10.4196/kjpp.2018.22.6.689.
Cyanidin-3-glucoside inhibits amyloid β₂₅₋₃₅-induced neuronal cell death in cultured rat hippocampal neurons
- Affiliations
-
- 1Department of Physiology, College of Medicine, Catholic Neuroscience Institute, The Catholic University of Korea, Seoul 06591, Korea. s-hyoon@catholic.ac.kr
- 2College of Pharmacy, The Catholic University of Korea, Bucheon 14662, Korea.
- KMID: 2430091
- DOI: http://doi.org/10.4196/kjpp.2018.22.6.689
Abstract
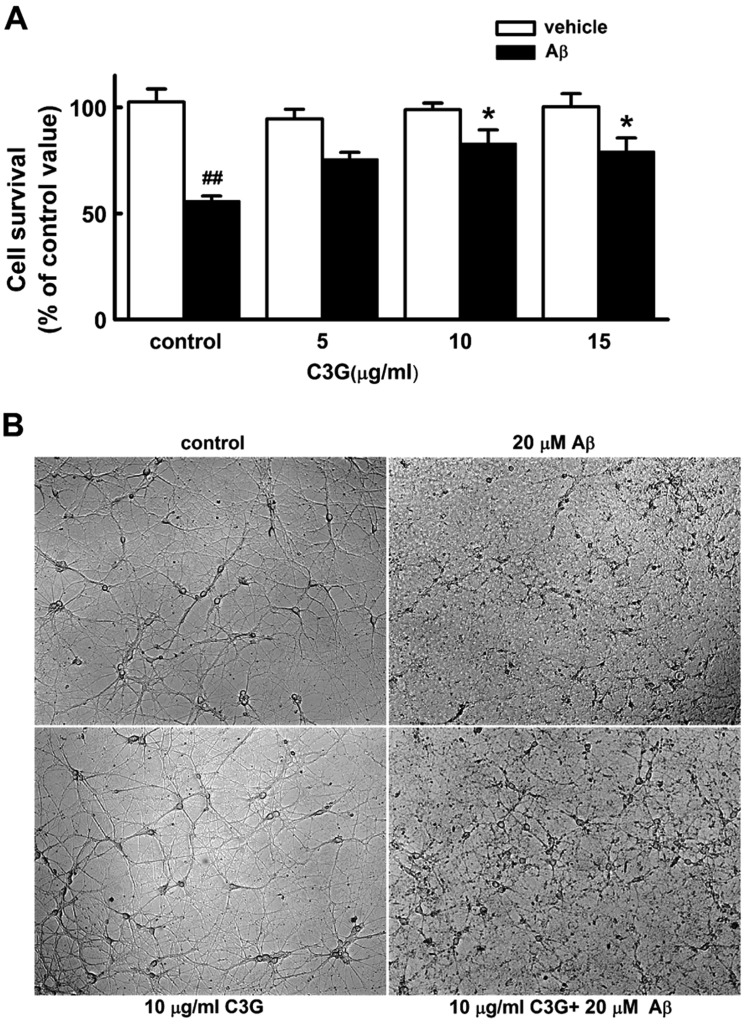
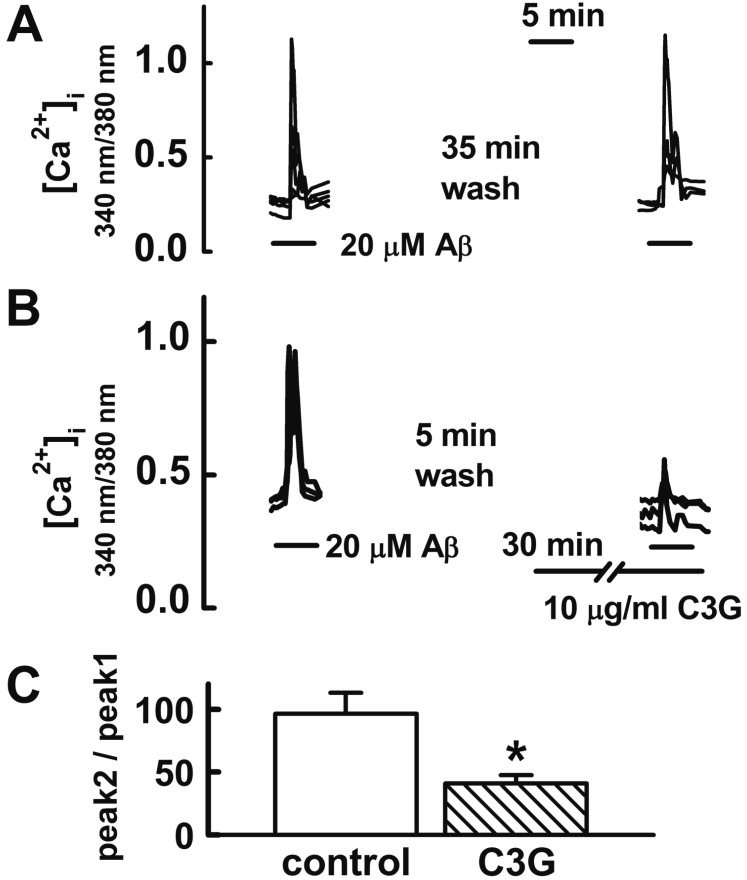
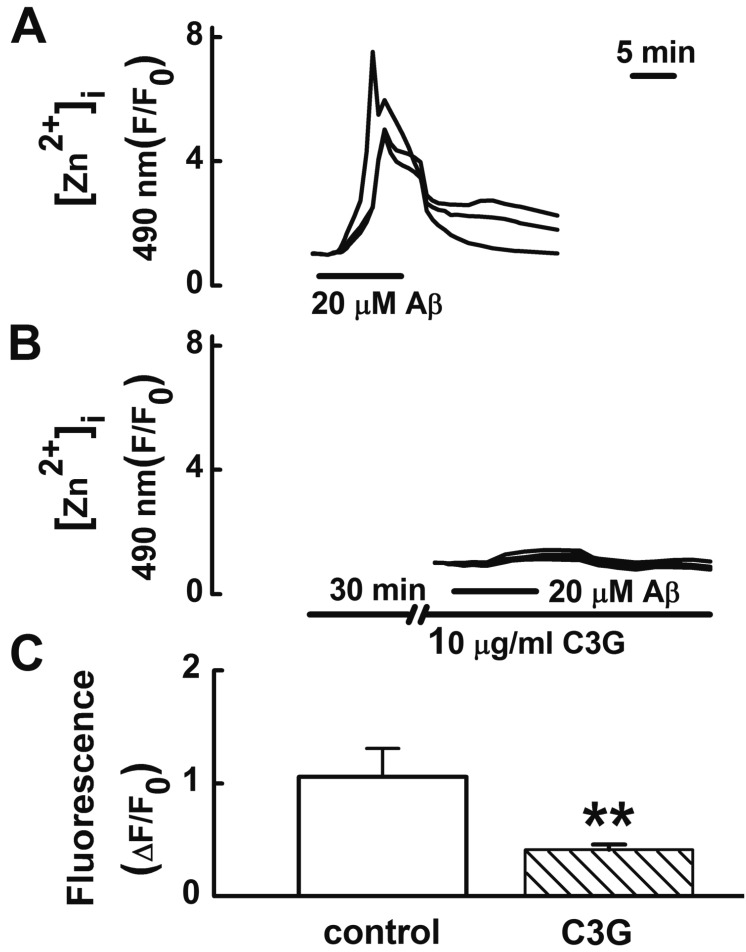
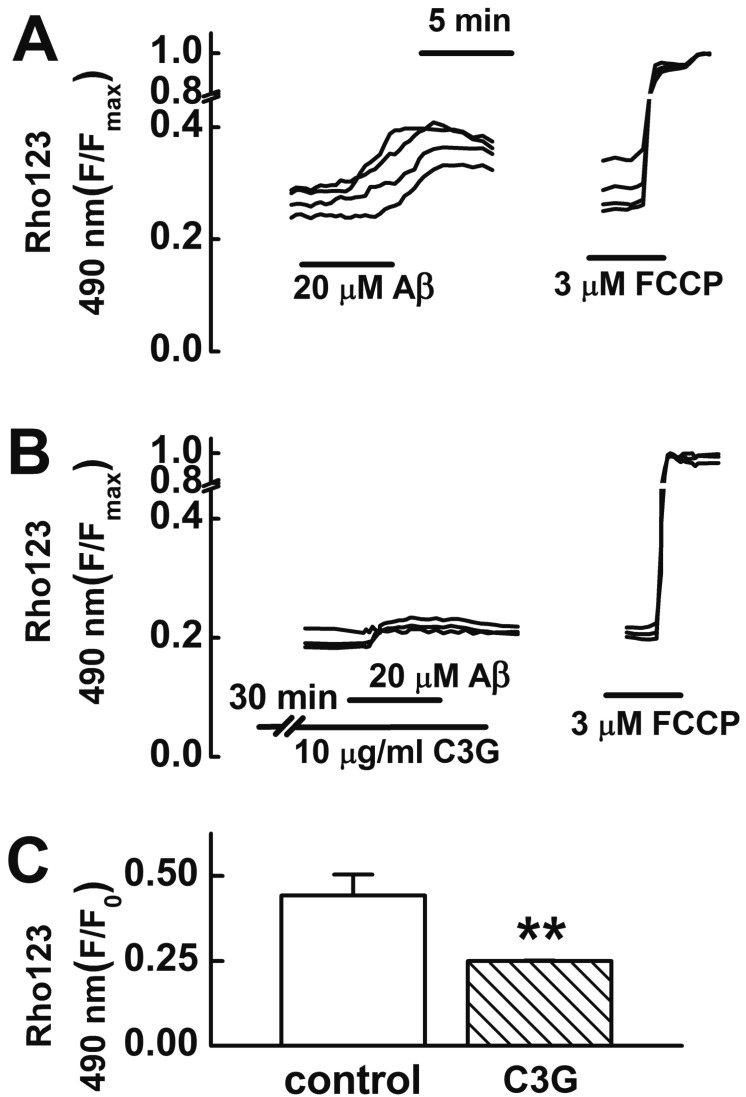
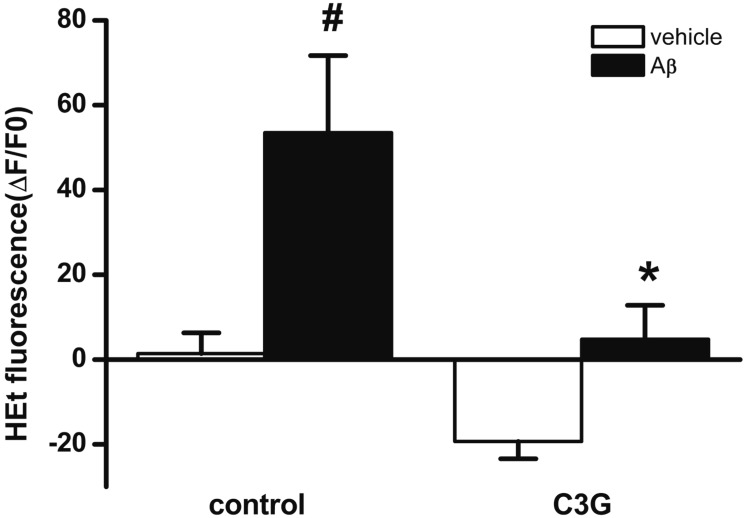
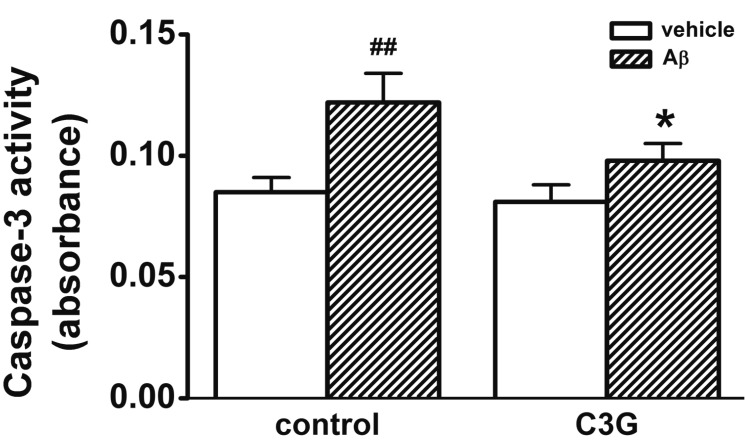
- Increasing evidence implicates changes in [Ca²âº]i and oxidative stress as causative factors in amyloid beta (Aβ)-induced neuronal cell death. Cyanidin-3-glucoside (C3G), a component of anthocyanin, has been reported to protect against glutamate-induced neuronal cell death by inhibiting Ca²âº and Zn²âº signaling. The present study aimed to determine whether C3G exerts a protective effect against Aβ₂₅₋₃₅-induced neuronal cell death in cultured rat hippocampal neurons from embryonic day 17 fetal Sprague-Dawley rats using MTT assay for cell survival, and caspase-3 assay and digital imaging methods for Ca²âº, Zn²âº, MMP and ROS. Treatment with Aβ25-35 (20 µM) for 48 h induced neuronal cell death in cultured rat pure hippocampal neurons. Treatment with C3G for 48 h significantly increased cell survival. Pretreatment with C3G for 30 min significantly inhibited Aβ₂₅₋₃₅-induced [Zn²âº]i increases as well as [Ca²âº]i increases in the cultured rat hippocampal neurons. C3G also significantly inhibited Aβ₂₅₋₃₅-induced mitochondrial depolarization. C3G also blocked the Aβ₂₅₋₃₅-induced formation of ROS. In addition, C3G significantly inhibited the Aβ₂₅₋₃₅-induced activation of caspase-3. These results suggest that cyanidin-3-glucoside protects against amyloid β-induced neuronal cell death by reducing multiple apoptotic signals.
Keyword
MeSH Terms
Figure
Reference
-
1. Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991; 30:572–580. PMID: 1789684.
Article2. Arispe N, Rojas E, Pollard HB. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci U S A. 1993; 90:567–571. PMID: 8380642.
Article3. Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004; 430:631–639. PMID: 15295589.
Article4. Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008; 31:454–463. PMID: 18675468.
Article5. Religa D, Strozyk D, Cherny RA, Volitakis I, Haroutunian V, Winblad B, Naslund J, Bush AI. Elevated cortical zinc in Alzheimer disease. Neurology. 2006; 67:69–75. PMID: 16832080.
Article6. Bush AI, Pettingell WH Jr, de Paradis M, Tanzi RE, Wasco W. The amyloid beta-protein precursor and its mammalian homologues. Evidence for a zinc-modulated heparin-binding superfamily. J Biol Chem. 1994; 269:26618–26621. PMID: 7929392.
Article7. Ahn SH, Kim HJ, Jeong I, Hong YJ, Kim MJ, Rhie DJ, Jo YH, Hahn SJ, Yoon SH. Grape seed proanthocyanidin extract inhibits glutamate-induced cell death through inhibition of calcium signals and nitric oxide formation in cultured rat hippocampal neurons. BMC Neurosci. 2011; 12:78. DOI: 10.1186/1471-2202-12-78. PMID: 21810275.
Article8. Bae JH, Mun KC, Park WK, Lee SR, Suh SI, Baek WK, Yim MB, Kwon TK, Song DK. EGCG attenuates AMPA-induced intracellular calcium increase in hippocampal neurons. Biochem Biophys Res Commun. 2002; 290:1506–1512. PMID: 11820792.9. Yang JS, Perveen S, Ha TJ, Kim SY, Yoon SH. Cyanidin-3-glucoside inhibits glutamate-induced Zn2+ signaling and neuronal cell death in cultured rat hippocampal neurons by inhibiting Ca2+-induced mitochondrial depolarization and formation of reactive oxygen species. Brain Res. 2015; 1606:9–20. PMID: 25721794.10. Bhuiyan MI, Kim HB, Kim SY, Cho KO. The Neuroprotective potential of cyanidin-3-glucoside fraction extracted from mulberry following oxygen-glucose deprivation. Korean J Physiol Pharmacol. 2011; 15:353–361. PMID: 22359473.
Article11. Kang TH, Hur JY, Kim HB, Ryu JH, Kim SY. Neuroprotective effects of the cyanidin-3-O-beta-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci Lett. 2006; 391:122–126. PMID: 16181734.12. Ke Z, Liu Y, Wang X, Fan Z, Chen G, Xu M, Bower KA, Frank JA, Ou X, Shi X, Luo J. Cyanidin-3-glucoside ameliorates ethanol neurotoxicity in the developing brain. J Neurosci Res. 2011; 89:1676–1684. PMID: 21671257.
Article13. Tarozzi A, Morroni F, Hrelia S, Angeloni C, Marchesi A, Cantelli-Forti G, Hrelia P. Neuroprotective effects of anthocyanins and their in vivo metabolites in SH-SY5Y cells. Neurosci Lett. 2007; 424:36–40. PMID: 17709193.
Article14. Tarozzi A, Merlicco A, Morroni F, Franco F, Cantelli-Forti G, Teti G, Falconi M, Hrelia P. Cyanidin 3-O-glucopyranoside protects and rescues SH-SY5Y cells against amyloid-beta peptide-induced toxicity. Neuroreport. 2008; 19:1483–1486. PMID: 18797302.
Article15. Kubo T, Nishimura S, Kumagae Y, Kaneko I. In vivo conversion of racemized beta-amyloid ([D-Ser 26]A beta 1-40) to truncated and toxic fragments ([D-Ser 26]A beta 25-35/40) and fragment presence in the brains of Alzheimer's patients. J Neurosci Res. 2002; 70:474–483. PMID: 12391608.16. Kim HJ, Kim TH, Choi SJ, Hong YJ, Yang JS, Sung KW, Rhie DJ, Hahn SJ, Yoon SH. Fluoxetine suppresses synaptically induced [Ca2+]i spikes and excitotoxicity in cultured rat hippocampal neurons. Brain Res. 2013; 1490:23–34. PMID: 23131584.17. Kim HJ, Martemyanov KA, Thayer SA. Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death. J Neurosci. 2008; 28:12604–12613. PMID: 19036954.
Article18. Dineley KE, Devinney MJ 2nd, Zeak JA, Rintoul GL, Reynolds IJ. Glutamate mobilizes [Zn2+] through Ca2+-dependent reactive oxygen species accumulation. J Neurochem. 2008; 106:2184–2193. PMID: 18624907.19. Agostinho P, Oliveira CR. Involvement of calcineurin in the neurotoxic effects induced by amyloid-beta and prion peptides. Eur J Neurosci. 2003; 17:1189–1196. PMID: 12670307.
Article20. Ferreiro E, Oliveira CR, Pereira C. Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5-triphosphate receptors in the neurotoxic effects induced by the amyloidbeta peptide. J Neurosci Res. 2004; 76:872–880. PMID: 15160398.21. Zeng H, Chen Q, Zhao B. Genistein ameliorates beta-amyloid peptide (25-35)-induced hippocampal neuronal apoptosis. Free Radic Biol Med. 2004; 36:180–188. PMID: 14744630.22. Resende R, Pereira C, Agostinho P, Vieira AP, Malva JO, Oliveira CR. Susceptibility of hippocampal neurons to Abeta peptide toxicity is associated with perturbation of Ca2+ homeostasis. Brain Res. 2007; 1143:11–21. PMID: 17336275.23. Perveen S, Yang JS, Ha TJ, Yoon SH. Cyanidin-3-glucoside inhibits ATP-induced intracellular free Ca2+ concentration, ROS formation and mitochondrial depolarization in PC12 cells. Korean J Physiol Pharmacol. 2014; 18:297–305. PMID: 25177161.24. Nasr P, Gursahani HI, Pang Z, Bondada V, Lee J, Hadley RW, Geddes JW. Influence of cytosolic and mitochondrial Ca2+, ATP, mitochondrial membrane potential, and calpain activity on the mechanism of neuron death induced by 3-nitropropionic acid. Neurochem Int. 2003; 43:89–99. PMID: 12620277.25. Sensi SL, Yin HZ, Weiss JH. AMPA/kainate receptor-triggered Zn2+ entry into cortical neurons induces mitochondrial Zn2+ uptake and persistent mitochondrial dysfunction. Eur J Neurosci. 2000; 12:3813–3818. PMID: 11029652.26. Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, Weiss JH. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci U S A. 2003; 100:6157–6162. PMID: 12724524.27. Pereira C, Santos MS, Oliveira C. Mitochondrial function impairment induced by amyloid beta-peptide on PC12 cells. Neuroreport. 1998; 9:1749–1755. PMID: 9665595.28. Gottlieb E, Vander Heiden MG, Thompson CB. Bcl-x(L) prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2000; 20:5680–5689. PMID: 10891504.29. Schubert D, Behl C, Lesley R, Brack A, Dargusch R, Sagara Y, Kimura H. Amyloid peptides are toxic via a common oxidative mechanism. Proc Natl Acad Sci U S A. 1995; 92:1989–1993. PMID: 7892213.
Article30. Bisaglia M, Venezia V, Piccioli P, Stanzione S, Porcile C, Russo C, Mancini F, Milanese C, Schettini G. Acetaminophen protects hippocampal neurons and PC12 cultures from amyloid beta-peptides induced oxidative stress and reduces NF-kappaB activation. Neurochem Int. 2002; 41:43–54. PMID: 11918971.31. Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004; 287:C817–C833. PMID: 15355853.
Article32. Oyama Y, Furukawa K, Chikahisa L, Hatakeyama Y. Effect of N,N-diethyldithiocarbamate on ionomycin-induced increase in oxidation of cellular 2′,7′-dichlorofluorescin in dissociated cerebellar neurons. Brain Res. 1994; 660:158–161. PMID: 7827993.
Article33. Adam-Vizi V, Starkov AA. Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J Alzheimers Dis. 2010; 20(Suppl 2):S413–S426. PMID: 20421693.
Article34. Thummayot S, Tocharus C, Pinkaew D, Viwatpinyo K, Sringarm K, Tocharus J. Neuroprotective effect of purple rice extract and its constituent against amyloid beta-induced neuronal cell death in SK-N-SH cells. Neurotoxicology. 2014; 45:149–158. PMID: 25451968.
Article35. Ullah I, Park HY, Kim MO. Anthocyanins protect against kainic acid-induced excitotoxicity and apoptosis via ROS-activated AMPK pathway in hippocampal neurons. CNS Neurosci Ther. 2014; 20:327–338. PMID: 24393263.
Article36. Mirzabekov T, Lin MC, Yuan WL, Marshall PJ, Carman M, Tomaselli K, Lieberburg I, Kagan BL. Channel formation in planar lipid bilayers by a neurotoxic fragment of the beta-amyloid peptide. Biochem Biophys Res Commun. 1994; 202:1142–1148. PMID: 7519420.37. Bondy SC, Guo-Ross SX, Truong AT. Promotion of transition metalinduced reactive oxygen species formation by beta-amyloid. Brain Res. 1998; 799:91–96. PMID: 9666089.38. Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI. The A beta peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999; 38:7609–7916. PMID: 10386999.39. Talavéra S, Felgines C, Texier O, Besson C, Gil-Izquierdo A, Lamaison JL, Rémésy C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J Agric Food Chem. 2005; 53:3902–3908. PMID: 15884815.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Neuroprotective Potential of Cyanidin-3-glucoside Fraction Extracted from Mulberry Following Oxygen-glucose Deprivation
- Protective effect of the aerial parts of Silybum marianum against amyloid β protein (25-35)-induced neuronal death in cultured neurons
- Cyanidin-3-glucoside Inhibits ATP-induced Intracellular Free Ca2+ Concentration, ROS Formation and Mitochondrial Depolarization in PC12 Cells
- Inhibitory effect of the leaves and stems of Actinidia arguta on Aβ (25–35)-induced neuronal cell death and memory impairment
- A TUNEL and Electron Microscopic Study on the Delayed Neuronal Death of Rat CA1 Pyramidal Neurons after MCAO