Allergy Asthma Immunol Res.
2019 Nov;11(6):806-817. 10.4168/aair.2019.11.6.806.
The Role of NF-κB in Chronic Rhinosinusitis With Nasal Polyps
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Chungbuk National University College of Medicine, Chungbuk National University Hospital, Cheongju, Korea.
- 2Center of Morphological Experiment, Medical College of Yanbian University, Yanji, China.
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Chuncheon Sacred Heart Hospital and Institute of New Frontier Research, Hallym University College of Medicine, Chuncheon, Korea.
- 4Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. dongkim@snu.ac.kr
- KMID: 2459185
- DOI: http://doi.org/10.4168/aair.2019.11.6.806
Abstract
- PURPOSE
Whereas the majority of nasal polyps observed in Western populations are eosinophilic, non-eosinophilic nasal polyps are significantly more frequent in Asian countries. Given the importance of nuclear factor-kappa B (NF-κB) in inflammation, this study focused on the role of NF-κB in the pathogenesis of chronic rhinosinusitis with nasal polyps (CRSwNPs) in Asian patients.
METHODS
A total of 46 patients were enrolled in this study (22 diagnosed with CRSwNPs, 10 with chronic rhinosinusitis without nasal polyps [CRSsNP], and 14 control subjects). Nasal polyps and uncinate tissues (UTs) were collected and the tissues prepared for hematoxylin-eosin staining and immunohistochemistric (IHC) analysis. Total RNA was isolated for real-time polymerase chain reaction for p65, interleukin (IL)-6, IL-8, intracellular adhesion molecule (ICAM)-1, IL-1β, tumor necrosis factor (TNF)-α, and eotaxin.
RESULTS
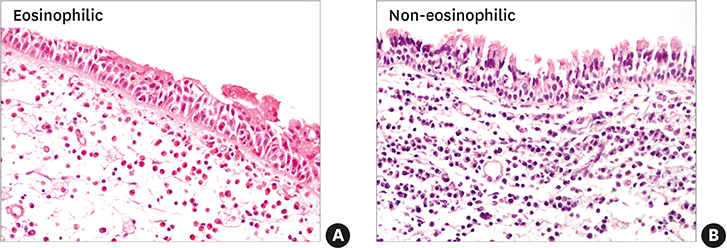
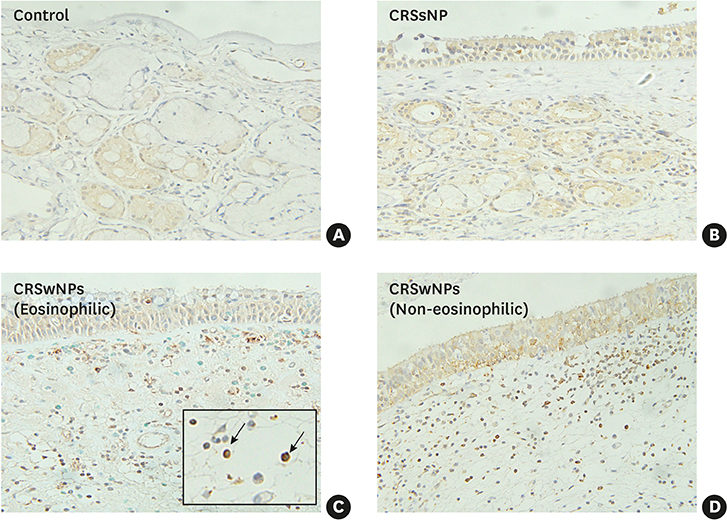
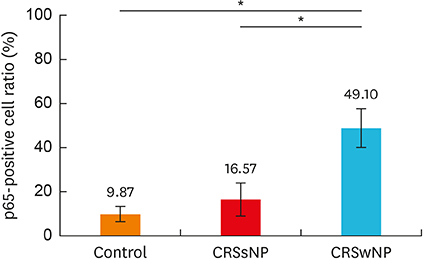
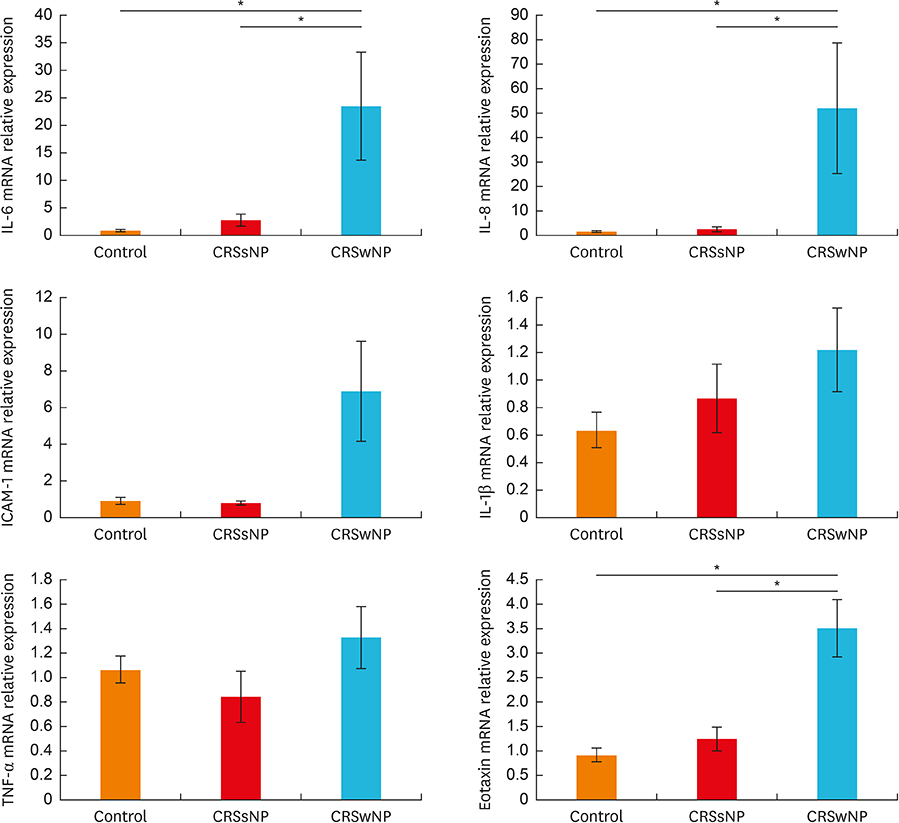
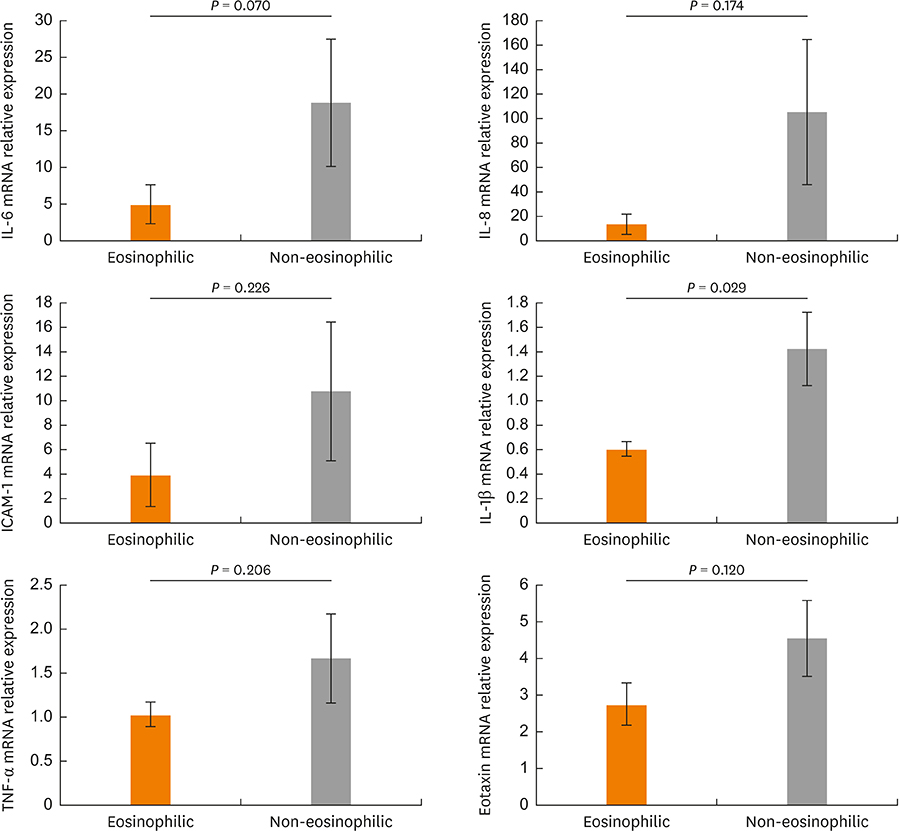
In the CRSwNPs group, 50% of nasal polyps were non-eosinophilic. IHC revealed a significantly higher fraction of NF-κB p65-positive cells in nasal polyps of the CRSwNPs group than in the UTs of control and CRSsNP groups. No difference in NF-κB p65-positive cell fraction was observed between eosinophilic and non-eosinophilic nasal polyps. The mRNA expression of p65, IL-6, IL-8, and eotaxin was significantly higher in nasal polyps of the CRSwNPs than in the UTs of control and CRSsNP group. However, no difference in expression was observed between eosinophilic and non-eosinophilic nasal polyps, with the exception of IL-1β expression.
CONCLUSIONS
Elevated expression of NF-κB- and NF-κB-associated inflammatory cytokines suggests NF-κB as the key factor for CRSwNPs pathogenesis in Asian patients. Understanding NF-κB-associated mechanisms will provide a deeper insight into CRSwNPs pathogenesis and ultimately improve therapeutic strategies for CRSwNPs.
MeSH Terms
-
Asian Continental Ancestry Group
Cytokines
Eosinophils
Humans
Immunohistochemistry
Interleukin-6
Interleukin-8
Interleukins
Nasal Polyps*
Real-Time Polymerase Chain Reaction
RNA
RNA, Messenger
Sinusitis
Transcription Factors
Tumor Necrosis Factor-alpha
Cytokines
Interleukin-6
Interleukin-8
Interleukins
RNA
RNA, Messenger
Transcription Factors
Tumor Necrosis Factor-alpha
Figure
Cited by 2 articles
-
Establishing a Therapeutic Strategy Targeting NF-κB in Asian Patients with Chronic Rhinosinusitis With Nasal Polyps
Young Hyo Kim
Allergy Asthma Immunol Res. 2019;11(6):757-759. doi: 10.4168/aair.2019.11.6.757.Critical Points on the Use of Biologicals in Allergic Diseases and Asthma
Ioana Agache, Catalina Cojanu, Alexandru Laculiceanu, Liliana Rogozea
Allergy Asthma Immunol Res. 2020;12(1):24-41. doi: 10.4168/aair.2020.12.1.24.
Reference
-
1. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012; 23:1–298.2. Pawankar R. Nasal polyposis: an update: editorial review. Curr Opin Allergy Clin Immunol. 2003; 3:1–6.3. Kim JW, Hong SL, Kim YK, Lee CH, Min YG, Rhee CS. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg. 2007; 137:925–930.
Article4. Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008; 122:961–968.
Article5. Soler ZM, Mace JC, Litvack JR, Smith TL. Chronic rhinosinusitis, race, and ethnicity. Am J Rhinol Allergy. 2012; 26:110–116.
Article6. Hao J, Pang YT, Wang DY. Diffuse mucosal inflammation in nasal polyps and adjacent middle turbinate. Otolaryngol Head Neck Surg. 2006; 134:267–275.
Article7. Gevaert P, Kaliner MA, Van Cauwenberge P. Global Resources in Allergy™. Chronic rhinosinusitis and nasal polyposis (module 10). Milwaukee (WI): World Allergy Organization;2011.8. Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004; 114:155–212.
Article9. Frączek M, Rostkowska-Nadolska B, Kapral M, Szota J, Kręcicki T, Mazurek U. Microarray analysis of NF-κB-dependent genes in chronic rhinosinusitis with nasal polyps. Adv Clin Exp Med. 2013; 22:209–217.10. Henriksson G, Norlander T, Forsgren J, Stierna P. Effects of topical budesonide treatment on glucocorticoid receptor mRNA down-regulation and cytokine patterns in nasal polyps. Am J Rhinol. 2001; 15:1–8.
Article11. Larsen K, Tos M. Clinical course of patients with primary nasal polyps. Acta Otolaryngol. 1994; 114:556–559.
Article12. Lund VJ. Diagnosis and treatment of nasal polyps. BMJ. 1995; 311:1411–1414.13. Vento SI, Ertama LO, Hytönen ML, Wolff CH, Malmberg CH. Nasal polyposis: clinical course during 20 years. Ann Allergy Asthma Immunol. 2000; 85:209–214.
Article14. Kirtsreesakul V. Nasal polyps: the relationship to allergy, sinonasal infection and histopathological type. J Med Assoc Thai. 2004; 87:277–282.15. Jareoncharsri P, Bunnag C, Muangsomboon S, Tunsuriyawong P, Assanasen P. Clinical and histopathological classification of nasal polyps in Thais. Siriraj Med J. 2002; 54:689–697.16. Tikaram A, Prepageran N. Asian nasal polyps: a separate entity? Med J Malaysia. 2013; 68:445–447.17. Daines SM, Orlandi RR. Inflammatory cytokines in allergy and rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010; 18:187–190.
Article18. Phan NT, Cabot PJ, Wallwork BD, Cervin AU, Panizza BJ. Cellular and molecular mechanisms of chronic rhinosinusitis and potential therapeutic strategies: review on cytokines, nuclear factor kappa B and transforming growth factor beta. J Laryngol Otol. 2015; 129:Suppl 3. S2–S7.
Article19. Adcock IM, Caramori G. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol Cell Biol. 2001; 79:376–384.
Article20. Takeno S, Hirakawa K, Ueda T, Furukido K, Osada R, Yajin K. Nuclear factor-kappa B activation in the nasal polyp epithelium: relationship to local cytokine gene expression. Laryngoscope. 2002; 112:53–58.
Article21. Xu R, Xu G, Shi J, Wen W. A correlative study of NF-κB activity and cytokines expression in human chronic nasal sinusitis. J Laryngol Otol. 2007; 121:644–649.
Article22. Lennard CM, Mann EA, Sun LL, Chang AS, Bolger WE. Interleukin-1 beta, interleukin-5, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in chronic sinusitis: response to systemic corticosteroids. Am J Rhinol. 2000; 14:367–373.23. Papon JF, Coste A, Gendron MC, Cordonnier C, Wingerstmann L, Peynègre R, et al. HLA-DR and ICAM-1 expression and modulation in epithelial cells from nasal polyps. Laryngoscope. 2002; 112:2067–2075.
Article24. Necela BM, Cidlowski JA. Mechanisms of glucocorticoid receptor action in noninflammatory and inflammatory cells. Proc Am Thorac Soc. 2004; 1:239–246.
Article25. Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997; 336:1066–1071.26. Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009; 1:a001651.
Article27. Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994; 12:141–179.28. Wilson SJ, Leone BA, Anderson D, Manning A, Holgate ST. Immunohistochemical analysis of the activation of NF-κB and expression of associated cytokines and adhesion molecules in human models of allergic inflammation. J Pathol. 1999; 189:265–272.
Article29. Silvestri M, Sabatini F, Scarso L, Cordone A, Dasic G, Rossi GA. Fluticasone propionate downregulates nasal fibroblast functions involved in airway inflammation and remodeling. Int Arch Allergy Immunol. 2002; 128:51–58.
Article30. Valera FC, Umezawa K, Brassesco MS, Castro-Gamero AM, Queiroz RG, Scrideli CA, et al. Suppression of inflammatory cytokine secretion by an NF-κB inhibitor DHMEQ in nasal polyps fibroblasts. Cell Physiol Biochem. 2012; 30:13–22.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medical treatment according to phenotypes of chronic rhinosinusitis
- Establishing a Therapeutic Strategy Targeting NF-κB in Asian Patients with Chronic Rhinosinusitis With Nasal Polyps
- The Role of Superantigen in Nasal Polypogenesis
- Role of Fungal and Bacterial Superantigen in the Pathogenesis of Chronic Rhinosinusitis with Polyps
- Tissue Remodeling in Rhinosinusitis