Korean J Orthod.
2019 Sep;49(5):299-309. 10.4041/kjod.2019.49.5.299.
Effects of pre-applied orthodontic force on the regeneration of periodontal tissues in tooth replantation
- Affiliations
-
- 1Private Practice, Gwangju, Korea.
- 2Department of Statistics, College of Natural Sciences, Chonnam National University, Gwangju, Korea.
- 3Department of Oral Anatomy, School of Dentistry, Chonnam National University, Gwangju, Korea.
- 4Dental Science Research Institute, Chonnam National University, Gwangju, Korea.
- 5Department of Orthodontics, School of Dentistry, Chonnam National University, Gwangju, Korea. jhcho@jnu.ac.kr
- 6Dental 4D Research Institute, Chonnam National University, Gwangju, Korea.
- KMID: 2458618
- DOI: http://doi.org/10.4041/kjod.2019.49.5.299
Abstract
OBJECTIVE
This study aimed to investigate the effect of pre-applied orthodontic force on the regeneration of periodontal ligament (PDL) tissues and the underlying mechanisms in tooth replantation.
METHODS
Orthodontic force (50 cN) was applied to the left maxillary first molars of 7-week-old male Sprague-Dawley rats (n = 32); the right maxillary first molars were left untreated to serve as the control group. After 7 days, the first molars on both sides were fully luxated and were immediately replanted in their original sockets. To verify the effects of the pre-applied orthodontic force, we assessed gene expression by using microarray analysis and real-time reverse transcription polymerase chain reaction (RT-PCR), cell proliferation by using proliferating cell nuclear antigen (PCNA) immunofluorescence staining, and morphological changes by using histological analysis.
RESULTS
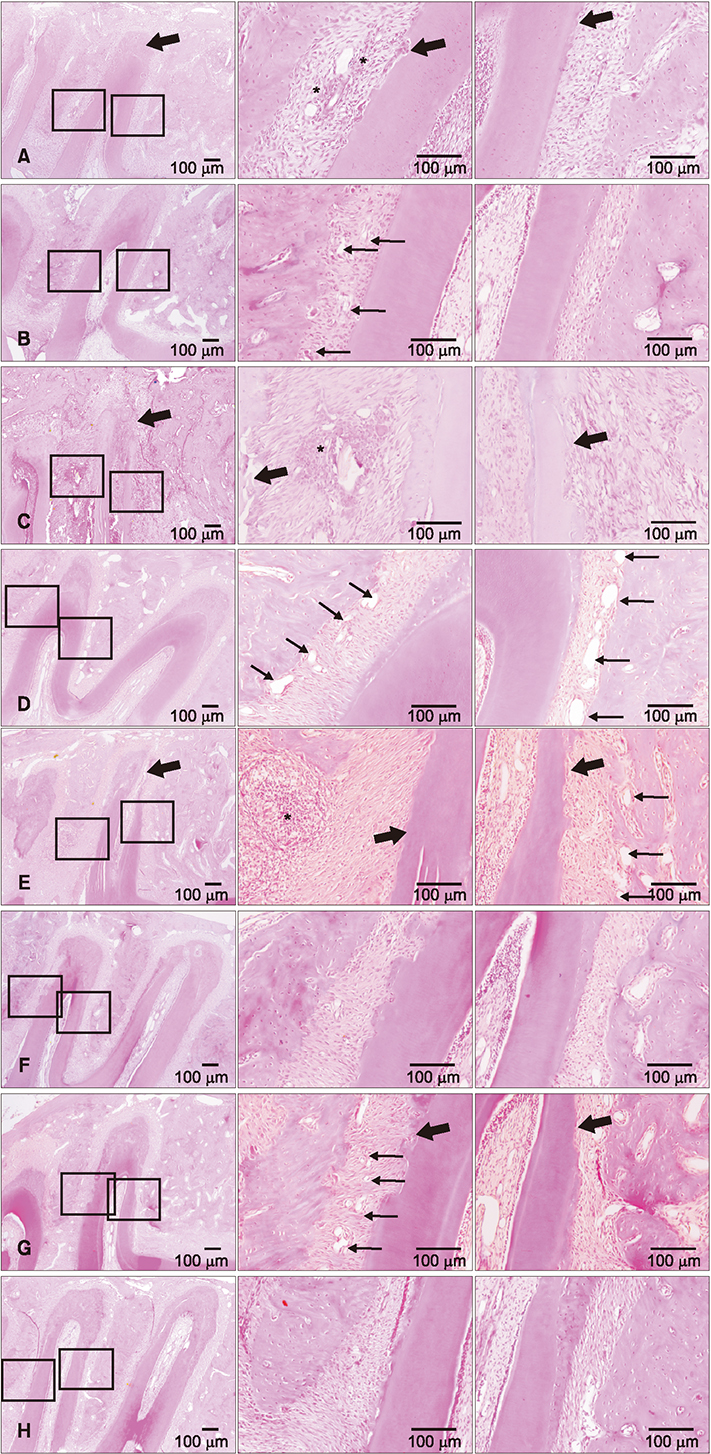
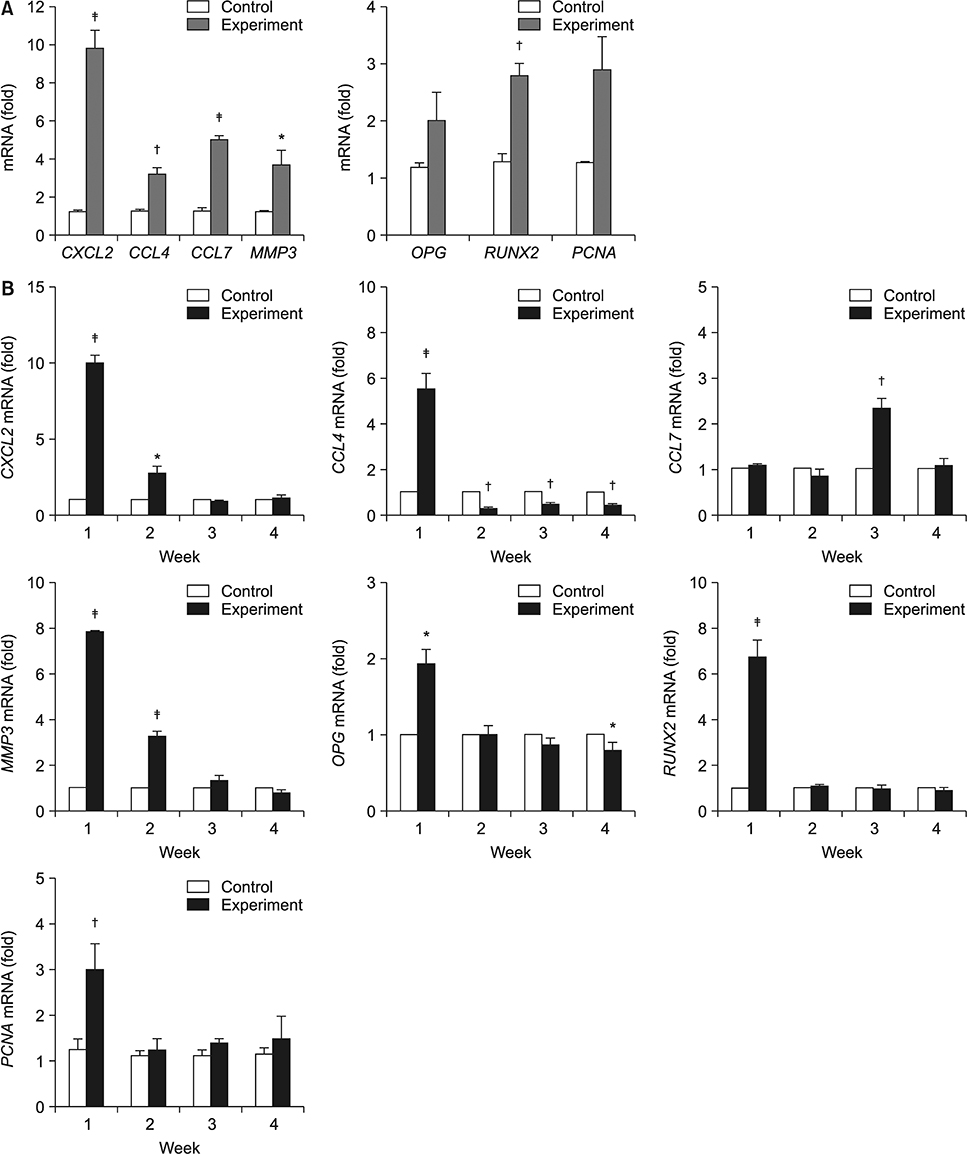
Application of orthodontic force for 7 days led to the proliferation of PDL tissues, as verified on microarray analysis and PCNA staining. Histological analysis after replantation revealed less root resorption, a better arrangement of PDL fibers, and earlier regeneration of periodontal tissues in the experimental group than in the control group. For the key genes involved in periodontal tissue remodeling, including CXCL2, CCL4, CCL7, MMP3, PCNA, OPG, and RUNX2, quantitative RT-PCR confirmed that messenger RNA levels were higher at 1 or 2 weeks in the experimental group.
CONCLUSIONS
These results suggest that the application of orthodontic force prior to tooth replantation enhanced the proliferation and activities of PDL cells and may lead to higher success rates with fewer complications.
MeSH Terms
-
Animals
Ankylosis
Cell Proliferation
Fluorescent Antibody Technique
Gene Expression
Humans
Male
Microarray Analysis
Molar
Periodontal Ligament
Polymerase Chain Reaction
Proliferating Cell Nuclear Antigen
Rats
Regeneration*
Replantation
Reverse Transcription
RNA, Messenger
Root Resorption
Tooth Replantation*
Tooth*
Proliferating Cell Nuclear Antigen
RNA, Messenger
Figure
Reference
-
1. Thilander B, Odman J, Lekholm U. Orthodontic aspects of the use of oral implants in adolescents: a 10-year follow-up study. Eur J Orthod. 2001; 23:715–731.
Article2. Schwartz O, Frederiksen K, Klausen B. Allotransplantation of human teeth. A retrospective study of 73 transplantations over a period of 28 years. Int J Oral Maxillofac Surg. 1987; 16:285–301.
Article3. Andreasen JO, Andersson L, Tsukiboshi M. Autotransplantation of teeth to the anterior region. In : Andreasen JO, Andreasen FM, Andreasen L, editors. Textbook and color atlas of traumatic injuries to the teeth. 4th ed. Copenhagen: Blackwell Munksgaard;2007. p. 740–760.4. Andreasen JO, Paulsen HU, Yu Z, Schwartz O. A long-term study of 370 autotransplanted premolars. Part III. Periodontal healing subsequent to transplantation. Eur J Orthod. 1990; 12:25–37.
Article5. Andreasen JO. Periodontal healing after replantation and autotransplantation of incisors in monkeys. Int J Oral Surg. 1981; 10:54–61.
Article6. Huang L, Liu B, Cha JY, Yuan G, Kelly M, Singh G, et al. Mechanoresponsive properties of the periodontal ligament. J Dent Res. 2016; 95:467–475.
Article7. Kalajzic Z, Peluso EB, Utreja A, Dyment N, Nihara J, Xu M, et al. Effect of cyclical forces on the periodontal ligament and alveolar bone remodeling during orthodontic tooth movement. Angle Orthod. 2014; 84:297–303.
Article8. Han J, Xu X, Zhang B, Chen B, Hang W. Expression of ATF4 and RUNX2 in periodontal tissue of pressure side during orthodontic tooth movement in rat. Int J Clin Exp Med. 2015; 8:934–938.9. Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, et al. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002; 30:e15.
Article10. Yang SY, Ko HM, Kang JH, Moon YH, Yoo HI, Jung NR, et al. Relaxin is up-regulated in the rat ovary by orthodontic tooth movement. Eur J Oral Sci. 2011; 119:115–120.
Article11. Andreasen JO. Analysis of pathogenesis and topography of replacement root resorption (ankylosis) after replantation of mature permanent incisors in monkeys. Swed Dent J. 1980; 4:231–240.12. Schwartz O, Andreasen FM, Andreasen JO. Effects of temperature, storage time and media on periodontal and pulpal healing after replantation of incisors in monkeys. Dent Traumatol. 2002; 18:190–195.
Article13. Oswald RJ, Harrington GW, Van Hassel HJ. Replantation 1. The role of the socket. J Endod. 1980; 6:479–484.
Article14. Mine K, Kanno Z, Muramoto T, Soma K. Occlusal forces promote periodontal healing of transplanted teeth and prevent dentoalveolar ankylosis: an experimental study in rats. Angle Orthod. 2005; 75:637–644.15. Andreasen JO. The effect of pulp extirpation or root canal treatment on periodontal healing after replantation of permanent incisors in monkeys. J Endod. 1981; 7:245–252.
Article16. Katayama A, Ota M, Sugito H, Shibukawa Y, Yamada S. Effect of proliferating tissue on transplanted teeth in dogs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:e110–e118.
Article17. Ren Y, Maltha JC, Kuijpers-Jagtman AM. The rat as a model for orthodontic tooth movement--a critical review and a proposed solution. Eur J Orthod. 2004; 26:483–490.
Article18. Tomizuka R, Shimizu Y, Kanetaka H, Suzuki A, Urayama S, Kikuchi M, et al. Histological evaluation of the effects of initially light and gradually increasing force on orthodontic tooth movement. Angle Orthod. 2007; 77:410–416.
Article19. Suzaki Y, Matsumoto Y, Kanno Z, Soma K. Preapplication of orthodontic forces to the donor teeth affects periodontal healing of transplanted teeth. Angle Orthod. 2008; 78:495–501.
Article20. Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003; 33:28–37.
Article21. Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res. 2007; 86:306–319.
Article22. Maeda A, Bandow K, Kusuyama J, Kakimoto K, Ohnishi T, Miyawaki S, et al. Induction of CXCL2 and CCL2 by pressure force requires IL-1β-MyD88 axis in osteoblasts. Bone. 2015; 74:76–82.
Article23. Ha J, Lee Y, Kim HH. CXCL2 mediates lipopolysaccharide-induced osteoclastogenesis in RANKL-primed precursors. Cytokine. 2011; 55:48–55.
Article24. Masella RS, Meister M. Current concepts in the biology of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2006; 129:458–468.
Article25. Madureira DF, Taddei Sde A, Abreu MH, Pretti H, Lages EM, da Silva TA. Kinetics of interleukin-6 and chemokine ligands 2 and 3 expression of periodontal tissues during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2012; 142:494–500.
Article26. Ishikawa K, Yoshida S, Nakao S, Sassa Y, Asato R, Kohno R, et al. Bone marrow-derived monocyte lineage cells recruited by MIP-1β promote physiological revascularization in mouse model of oxygen-induced retinopathy. Lab Invest. 2012; 92:91–101.
Article27. Taub DD, Proost P, Murphy WJ, Anver M, Longo DL, van Damme J, et al. Monocyte chemotactic protein-1 (MCP-1), -2, and -3 are chemotactic for human T lymphocytes. J Clin Invest. 1995; 95:1370–1376.
Article28. Ben-Baruch A, Xu L, Young PR, Bengali K, Oppenheim JJ, Wang JM. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors. C-C CKR1, a receptor for macrophage inflammatory protein-1 alpha/Rantes, is also a functional receptor for MCP3. J Biol Chem. 1995; 270:22123–22128.
Article29. Beklen A, Tüter G, Sorsa T, Hanemaaijer R, Virtanen I, Tervahartiala T, et al. Gingival tissue and crevicular fluid co-operation in adult periodontitis. J Dent Res. 2006; 85:59–63.
Article30. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997; 89:747–754.
Article31. Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010; 28:357–364.
Article32. Liu TM, Lee EH. Transcriptional regulatory cascades in Runx2-dependent bone development. Tissue Eng Part B Rev. 2013; 19:254–263.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intentional replantation with preapplication of orthodontic force on mandibular second molar
- Intrusion of the extruded maxillary central incisor using skeletal anchorage system and unilateral segmental intrusion arch
- Mode of tooth movement according to the timing of orthodontic force application after extraction
- Treatment of a tooth with severe periodontal involvement using intentional replantation: case report
- The study on the periodontal vascular changes of rat incisors following experimental tooth movement