Cancer Res Treat.
2019 Jul;51(3):1167-1179. 10.4143/crt.2018.526.
Therapeutic Targeting of the DNA Damage Response Using an ATR Inhibitor in Biliary Tract Cancer
- Affiliations
-
- 1Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. ohdoyoun@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- KMID: 2454308
- DOI: http://doi.org/10.4143/crt.2018.526
Abstract
- PURPOSE
The DNA damage response (DDR) is a multi-complex network of signaling pathways involved in DNA damage repair, cell cycle checkpoints, and apoptosis. In the case of biliary tract cancer (BTC), the strategy of DDR targeting has not been evaluated, even though many patients have DNA repair pathway alterations. The purpose of this study was to test the DDR-targeting strategy in BTC using an ataxia-telangiectasia and Rad3-related (ATR) inhibitor.
MATERIALS AND METHODS
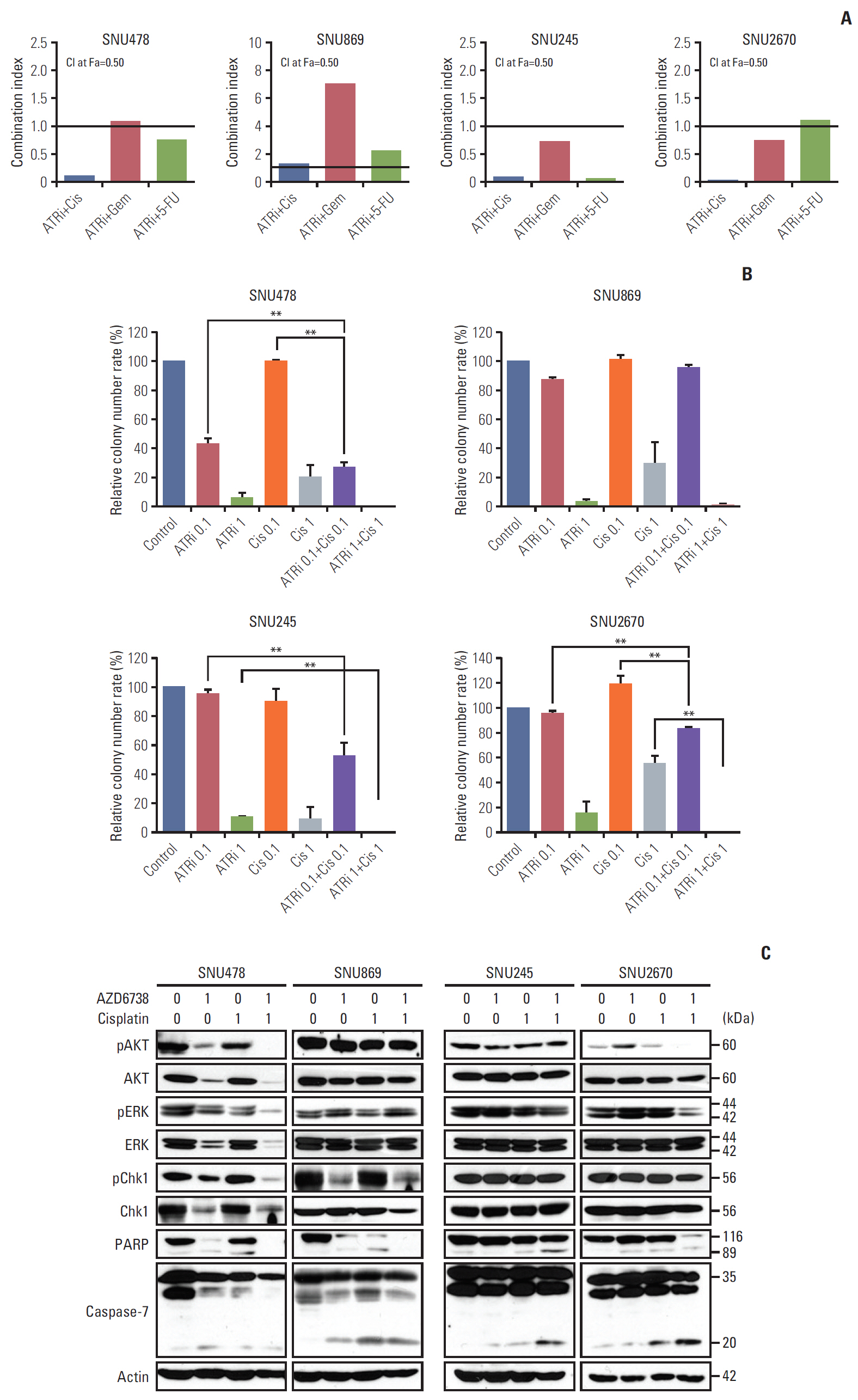
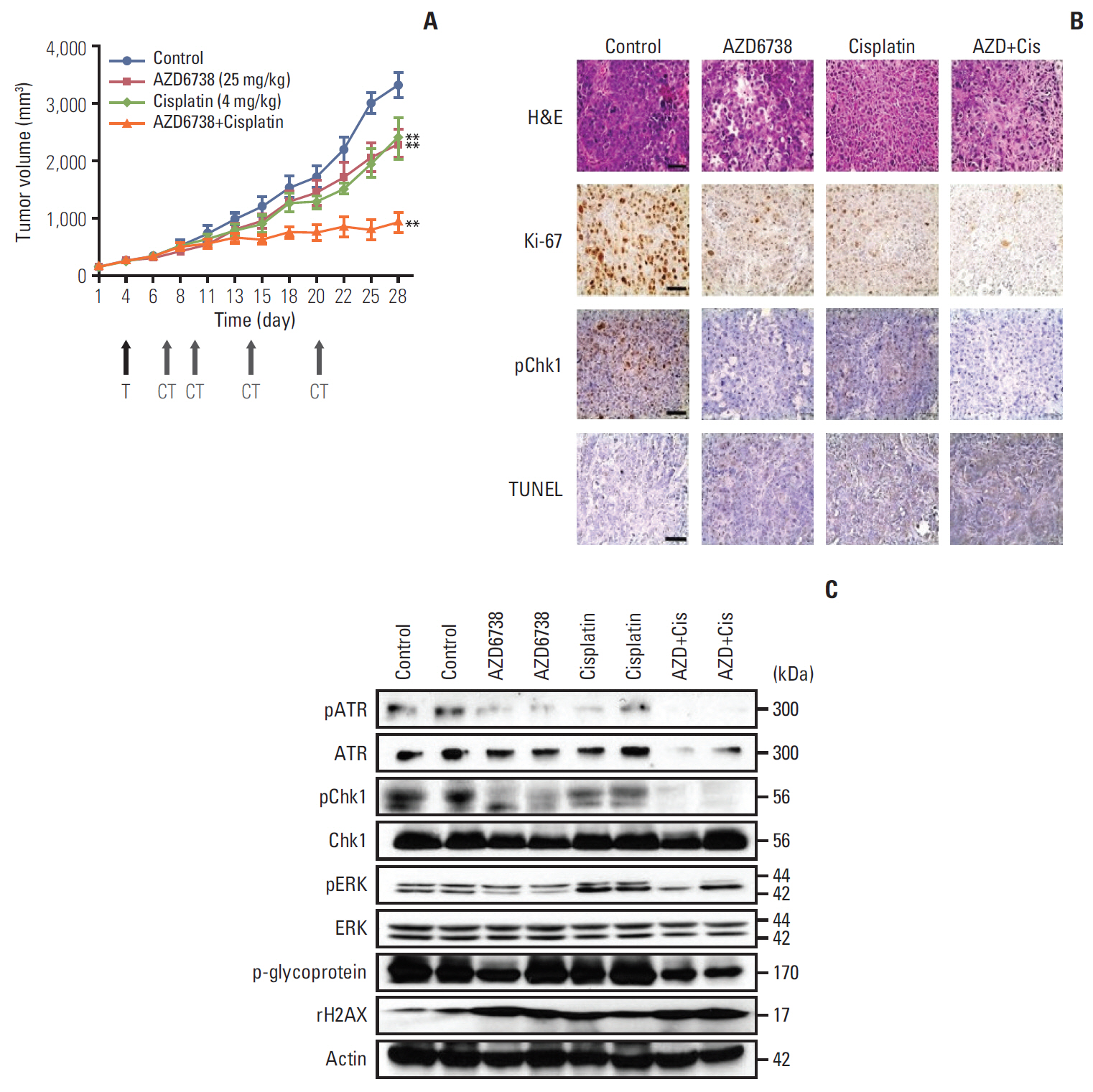
A total of nine human BTC cell lines were used for evaluating anti-tumor effect of AZD6738 (ATR inhibitor) alone or combination with cytotoxic chemotherapeutic agents through MTT assay, colony-forming assays, cell cycle analyses, and comet assays. We established SNU478-mouse model for in vivo experiments to confirm our findings.
RESULTS
Among nine human BTC cell lines, SNU478 and SNU869 were the most sensitive to AZD6738, and showed low expression of both ataxia-telangiectasia mutated (ATM) and p53. AZD6738 blocked p-Chk1 and p-glycoprotein and increased γH2AX, a marker of DNA damage, in sensitive cells. AZD6738 significantly increased apoptosis, G2/M arrest and p21, and decreased CDC2. Combinations of AZD6738 and cytotoxic chemotherapeutic agents exerted synergistic effects in colony-forming assays, cell cycle analyses, and comet assays. In our mouse models, AZD6738 monotherapy decreased tumor growth and the combination with cisplatin showed more potent effects on growth inhibition, decreased Ki-67, and increased terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling than monotherapy with each drug.
CONCLUSION
In BTC, DDR targeting strategy using ATR inhibitor demonstrated promising antitumor activity alone or in combination with cytotoxic chemotherapeutic agents. This supports further clinical development of DDR targeting strategy in BTC.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Inhibition of ATR Increases the Sensitivity to WEE1 Inhibitor in Biliary Tract Cancer
Ah-Rong Nam, Mei-Hua Jin, Ju-Hee Bang, Kyoung-Seok Oh, Hye-Rim Seo, Do-Youn Oh, Yung-Jue Bang
Cancer Res Treat. 2020;52(3):945-956. doi: 10.4143/crt.2020.080.Inhibition of WEE1 Potentiates Sensitivity to PARP Inhibitor in Biliary Tract Cancer
Hye-Rim Seo, Ah-Rong Nam, Ju-Hee Bang, Kyoung-Seok Oh, Jae-Min Kim, Jeesun Yoon, Tae-Yong Kim, Do-Youn Oh
Cancer Res Treat. 2022;54(2):541-553. doi: 10.4143/crt.2021.473.
Reference
-
References
1. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016; 13:261–80.2. O'Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015; 60:547–60.3. Pearl LH, Schierz AC, Ward SE, Al-Lazikani B, Pearl FM. Therapeutic opportunities within the DNA damage response. Nat Rev Cancer. 2015; 15:166–80.
Article4. Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer. 2012; 12:801–17.
Article5. Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 2008; 9:297–308.
Article6. Zou L. Single- and double-stranded DNA: building a trigger of ATR-mediated DNA damage response. Genes Dev. 2007; 21:879–85.7. Buisson R, Boisvert JL, Benes CH, Zou L. Distinct but concerted roles of ATR, DNA-PK, and Chk1 in countering replication stress during S phase. Mol Cell. 2015; 59:1011–24.8. Eykelenboom JK, Harte EC, Canavan L, Pastor-Peidro A, Calvo-Asensio I, Llorens-Agost M, et al. ATR activates the S-M checkpoint during unperturbed growth to ensure sufficient replication prior to mitotic onset. Cell Rep. 2013; 5:1095–107.
Article9. Weber AM, Ryan AJ. ATM and ATR as therapeutic targets in cancer. Pharmacol Ther. 2015; 149:124–38.
Article10. Karnitz LM, Zou L. Molecular pathways: targeting ATR in cancer therapy. Clin Cancer Res. 2015; 21:4780–5.
Article11. Foote KM, Lau A, Nissink JW. Drugging ATR: progress in the development of specific inhibitors for the treatment of cancer. Future Med Chem. 2015; 7:873–91.
Article12. Thanan R, Pairojkul C, Pinlaor S, Khuntikeo N, Wongkham C, Sripa B, et al. Inflammation-related DNA damage and expression of CD133 and Oct3/4 in cholangiocarcinoma patients with poor prognosis. Free Radic Biol Med. 2013; 65:1464–72.
Article13. Moy AP, Shahid M, Ferrone CR, Borger DR, Zhu AX, Ting D, et al. Microsatellite instability in gallbladder carcinoma. Virchows Arch. 2015; 466:393–402.
Article14. Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015; 47:1003–10.
Article15. Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One. 2014; 9:e115383.
Article16. Bouwman P, Jonkers J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer. 2012; 12:587–98.
Article17. Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014; 25:304–17.
Article18. Reaper PM, Griffiths MR, Long JM, Charrier JD, Maccormick S, Charlton PA, et al. Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nat Chem Biol. 2011; 7:428–30.
Article19. Nam AR, Kim JW, Cha Y, Ha H, Park JE, Bang JH, et al. Therapeutic implication of HER2 in advanced biliary tract cancer. Oncotarget. 2016; 7:58007–21.
Article20. Kim HJ, Min A, Im SA, Jang H, Lee KH, Lau A, et al. Antitumor activity of the ATR inhibitor AZD6738 in HER2 positive breast cancer cells. Int J Cancer. 2017; 140:109–19.
Article21. Min A, Im SA, Jang H, Kim S, Lee M, Kim DK, et al. AZD6738, a novel oral inhibitor of ATR, induces synthetic lethality with ATM deficiency in gastric cancer cells. Mol Cancer Ther. 2017; 16:566–77.
Article22. George E, Kim H, Krepler C, Wenz B, Makvandi M, Tanyi JL, et al. A patient-derived-xenograft platform to study BRCA-deficient ovarian cancers. JCI Insight. 2017; 2:e89760.
Article23. Ruiz S, Mayor-Ruiz C, Lafarga V, Murga M, Vega-Sendino M, Ortega S, et al. A genome-wide CRISPR screen identifies CDC25A as a determinant of sensitivity to ATR inhibitors. Mol Cell. 2016; 62:307–13.
Article24. Williamson CT, Miller R, Pemberton HN, Jones SE, Campbell J, Konde A, et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat Commun. 2016; 7:13837.
Article25. Mohni KN, Kavanaugh GM, Cortez D. ATR pathway inhibition is synthetically lethal in cancer cells with ERCC1 deficiency. Cancer Res. 2014; 74:2835–45.
Article26. Gunaydin H, Weiss MM, Sun Y. De novo prediction of p-glycoprotein-mediated efflux liability for druglike compounds. ACS Med Chem Lett. 2013; 4:108–12.
Article27. Li CC, Yang JC, Lu MC, Lee CL, Peng CY, Hsu WY, et al. ATRChk1 signaling inhibition as a therapeutic strategy to enhance cisplatin chemosensitivity in urothelial bladder cancer. Oncotarget. 2016; 7:1947–59.
Article28. Buisson R, Niraj J, Rodrigue A, Ho CK, Kreuzer J, Foo TK, et al. Coupling of homologous recombination and the checkpoint by ATR. Mol Cell. 2017; 65:336–46.
Article29. Sangster-Guity N, Conrad BH, Papadopoulos N, Bunz F. ATR mediate cisplatin resistance in a p53 genotype-specific manner. Oncogene. 2011; 30:2526–33.30. Vendetti FP, Lau A, Schamus S, Conrads TP, O'Connor MJ, Bakkenist CJ. The orally active and bioavailable ATR kinase inhibitor AZD6738 potentiates the anti-tumor effects of cisplatin to resolve ATM-deficient non-small cell lung cancer in vivo. Oncotarget. 2015; 6:44289–305.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of ATR Increases the Sensitivity to WEE1 Inhibitor in Biliary Tract Cancer
- Therapeutic Co-targeting of WEE1 and ATM Downregulates PD-L1 Expression in Pancreatic Cancer
- Inhibition of WEE1 Potentiates Sensitivity to PARP Inhibitor in Biliary Tract Cancer
- Aberrant DNA Double-strand Break Repair Threads in Breast Carcinoma: Orchestrating Genomic Insult Survival
- Molecular Aspects of Radiotherapy