Cancer Res Treat.
2022 Apr;54(2):541-553. 10.4143/crt.2021.473.
Inhibition of WEE1 Potentiates Sensitivity to PARP Inhibitor in Biliary Tract Cancer
- Affiliations
-
- 1Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 2Integrated Major in Innovative Medical Science, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- KMID: 2528223
- DOI: http://doi.org/10.4143/crt.2021.473
Abstract
- Purpose
Up to 20% of patients with biliary tract cancer (BTC) have alterations in DNA damage response (DDR) genes, including homologous recombination (HR) genes. Therefore, the DDR pathway could be a promising target for new drug development in BTC. We aim to investigate the anti-tumor effects using poly(ADP-ribose) polymerase (PARP) and WEE1 inhibitors in BTC.
Materials and Methods
We used 10 BTC cell lines to evaluate an anti-tumor effect of olaparib (a PARP inhibitor) and AZD1775 (a WEE1 inhibitor) in in vitro. Additionally, we established SNU869 xenograft model for in vivo experiments.
Results
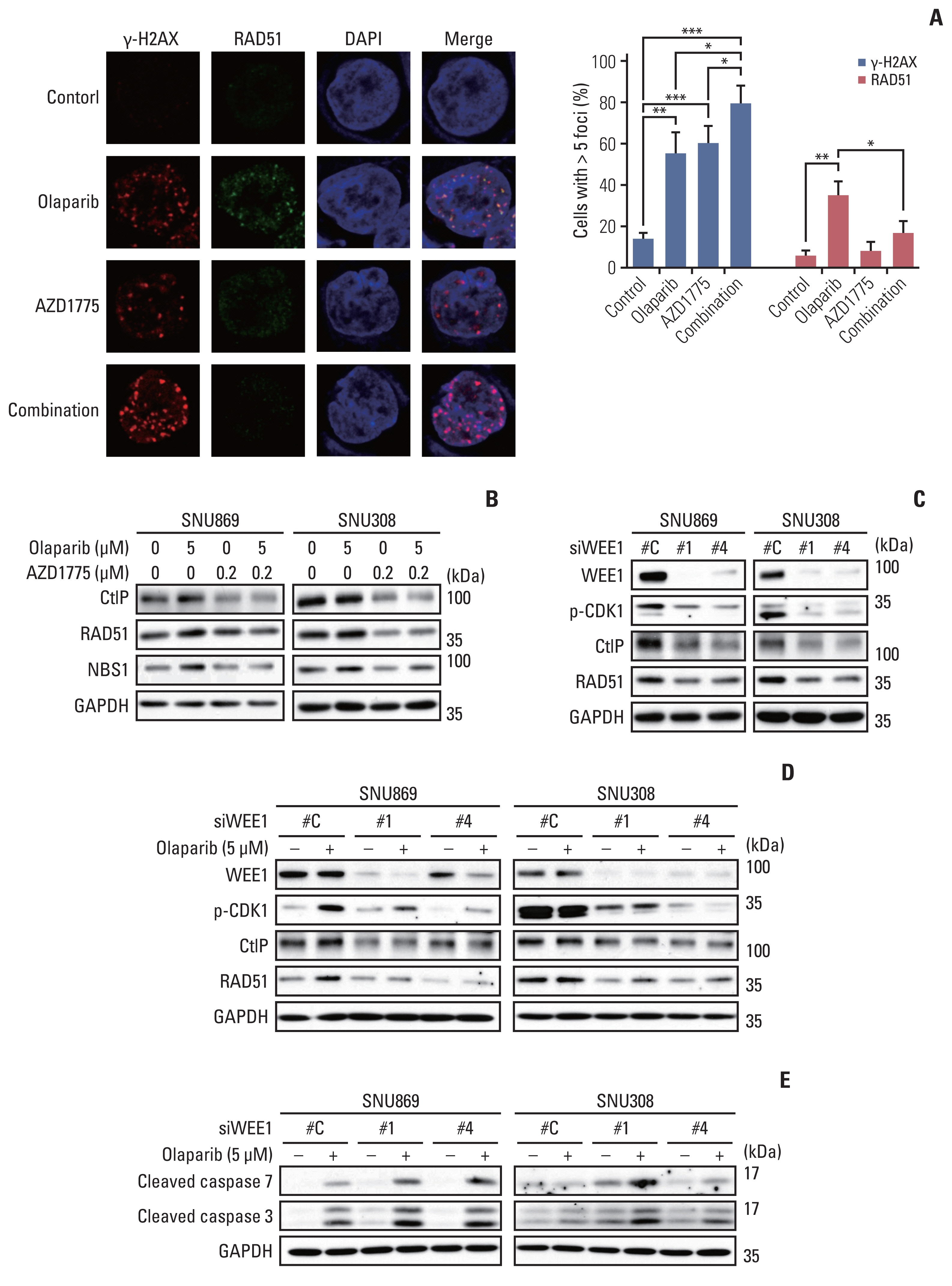
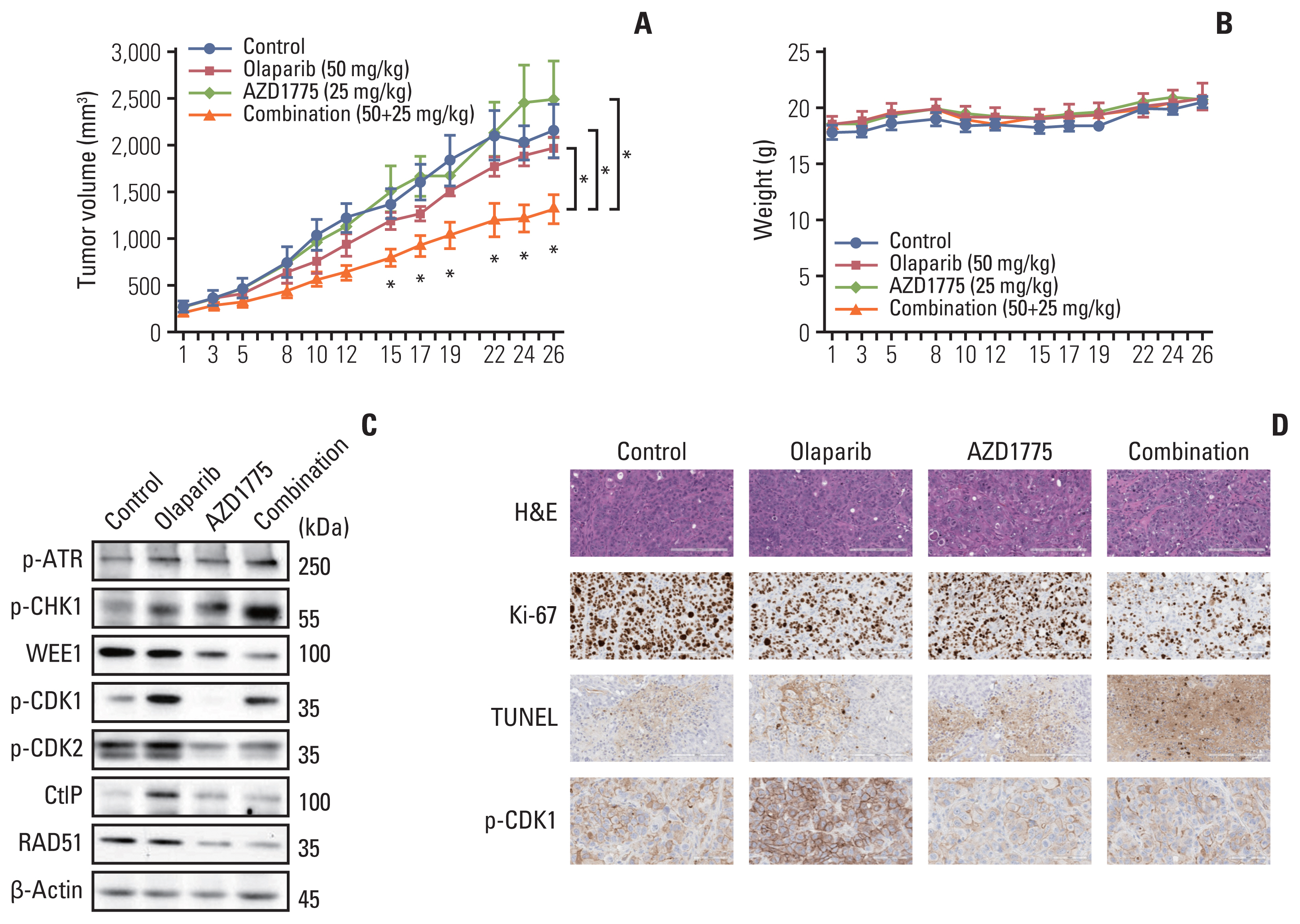
In this study, we observed a modest anti-proliferative effect of olaparib. DNA double-strand break (DSB) and apoptosis were increased by olaparib in BTC cells. However, olaparib-induced DNA DSB was repaired through the HR pathway, and G2 arrest was induced to secure the time for repair. As AZD1775 typically regulates the G2/M checkpoint, we combined olaparib with AZD1775 to abrogate G2 arrest. We observed that AZD1775 downregulated p-CDK1, a G2/M cell cycle checkpoint protein, and induced early mitotic entry. AZD1775 also decreased CtIP and RAD51 expression and disrupted HR repair. In xenograft model, olaparib plus AZD1775 treatment reduced tumor growth more potently than did monotherapy with either drug.
Conclusion
This is the first study to suggest that olaparib combined with AZD1775 can induce synergistic anti-tumor effects against BTC. Combination therapy that blocks dual PARP and WEE1 has the potential to be further clinically developed for BTC patients.
Keyword
Figure
Reference
-
References
1. Gottifredi V. Targeting DNA damage response kinases in cancer therapy. Mutat Res. 2020; 821:111725.
Article2. Tariq NU, McNamara MG, Valle JW. Biliary tract cancers: current knowledge, clinical candidates and future challenges. Cancer Manag Res. 2019; 11:2623–42.3. Ricci AD, Rizzo A, Bonucci C, Tober N, Palloni A, Mollica V, et al. PARP inhibitors in biliary tract cancer: a new kid on the block? Medicines (Basel). 2020; 7:54.
Article4. Ahn DH, Bekaii-Saab T. Biliary tract cancer and genomic alterations in homologous recombinant deficiency: exploiting synthetic lethality with PARP inhibitors. Chin Clin Oncol. 2020; 9:6.
Article5. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017; 377:523–33.6. Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019; 381:317–27.
Article7. Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018; 379:2495–505.
Article8. de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020; 382:2091–102.
Article9. Pommier Y, O’Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med. 2016; 8:362ps17.
Article10. Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008; 18:134–47.
Article11. Sunada S, Nakanishi A, Miki Y. Crosstalk of DNA double-strand break repair pathways in poly(ADP-ribose) polymerase inhibitor treatment of breast cancer susceptibility gene 1/2-mutated cancer. Cancer Sci. 2018; 109:893–9.
Article12. Lee EK, Matulonis UA. PARP inhibitor resistance mechanisms and implications for post-progression combination therapies. Cancers (Basel). 2020; 12:2054.
Article13. Jelinic P, Levine DA. New insights into PARP inhibitors’ effect on cell cycle and homology-directed DNA damage repair. Mol Cancer Ther. 2014; 13:1645–54.
Article14. Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001; 20:1803–15.
Article15. Patil M, Pabla N, Dong Z. Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell Mol Life Sci. 2013; 70:4009–21.
Article16. Ghelli Luserna di Rora A, Cerchione C, Martinelli G, Simonetti G. A WEE1 family business: regulation of mitosis, cancer progression, and therapeutic target. J Hematol Oncol. 2020; 13:126.
Article17. Schmidt M, Rohe A, Platzer C, Najjar A, Erdmann F, Sippl W. Regulation of G2/M transition by inhibition of WEE1 and PKMYT1 kinases. Molecules. 2017; 22:2045.
Article18. Yang L, Shen C, Pettit CJ, Li T, Hu AJ, Miller ED, et al. Wee1 kinase inhibitor AZD1775 effectively sensitizes esophageal cancer to radiotherapy. Clin Cancer Res. 2020; 26:3740–50.
Article19. Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004; 23:2825–37.
Article20. Krajewska M, Heijink AM, Bisselink YJ, Seinstra RI, Sillje HH, de Vries EG, et al. Forced activation of Cdk1 via wee1 inhibition impairs homologous recombination. Oncogene. 2013; 32:3001–8.
Article21. Nam AR, Kim JW, Cha Y, Ha H, Park JE, Bang JH, et al. Therapeutic implication of HER2 in advanced biliary tract cancer. Oncotarget. 2016; 7:58007–21.
Article22. Nam AR, Jin MH, Park JE, Bang JH, Oh DY, Bang YJ. Therapeutic targeting of the DNA damage response using an ATR inhibitor in biliary tract cancer. Cancer Res Treat. 2019; 51:1167–79.
Article23. Geenen JJ, Schellens JH. Molecular pathways: targeting the protein kinase Wee1 in cancer. Clin Cancer Res. 2017; 23:4540–4.
Article24. Liu K, Zheng M, Lu R, Du J, Zhao Q, Li Z, et al. The role of CDC25C in cell cycle regulation and clinical cancer therapy: a systematic review. Cancer Cell Int. 2020; 20:213.
Article25. Wang J, Ding Q, Fujimori H, Motegi A, Miki Y, Masutani M. Loss of CtIP disturbs homologous recombination repair and sensitizes breast cancer cells to PARP inhibitors. Oncotarget. 2016; 7:7701–14.
Article26. Zhao Q, Guan J, Zhang Z, Lv J, Wang Y, Liu L, et al. Inhibition of Rad51 sensitizes breast cancer cells with wild-type PTEN to olaparib. Biomed Pharmacother. 2017; 94:165–8.
Article27. Lim G, Chang Y, Huh WK. Phosphoregulation of Rad51/ Rad52 by CDK1 functions as a molecular switch for cell cycle-specific activation of homologous recombination. Sci Adv. 2020; 6:eaay2669.28. Buis J, Stoneham T, Spehalski E, Ferguson DO. Mre11 regulates CtIP-dependent double-strand break repair by interaction with CDK2. Nat Struct Mol Biol. 2012; 19:246–52.
Article29. Wang H, Shi LZ, Wong CC, Han X, Hwang PY, Truong LN, et al. The interaction of CtIP and Nbs1 connects CDK and ATM to regulate HR-mediated double-strand break repair. PLoS Genet. 2013; 9:e1003277.
Article30. Nam AR, Jin MH, Bang JH, Oh KS, Seo HR, Oh DY, et al. Inhibition of ATR increases the sensitivity to WEE1 inhibitor in biliary tract cancer. Cancer Res Treat. 2020; 52:945–56.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Exploiting DNA replication stress for sequential therapy with PARP and WEE1 inhibitors
- Inhibition of ATR Increases the Sensitivity to WEE1 Inhibitor in Biliary Tract Cancer
- Major clinical research advances in gynecologic cancer in 2023: a tumultuous year for endometrial cancer
- Therapeutic Co-targeting of WEE1 and ATM Downregulates PD-L1 Expression in Pancreatic Cancer
- The Important Role of Poly ADP-Ribose Polymerase Inhibitor in Prostate Cancer