Cancer Res Treat.
2019 Jul;51(3):1128-1134. 10.4143/crt.2018.379.
A Single Arm, Phase II Study of Simvastatin Plus XELOX and Bevacizumab as First-Line Chemotherapy in Metastatic Colorectal Cancer Patients
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. wkkang@skku.edu
- 2Division of Hematology-Oncology, Department of Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine Changwon, Korea.
- 3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Institute for Cancer Research, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2454304
- DOI: http://doi.org/10.4143/crt.2018.379
Abstract
- PURPOSE
Simvastatin has demonstrated anti-tumor activity in preclinical studies via tumor cell senescence, apoptosis, and anti-angiogenesis. This phase II trial evaluated the efficacy and toxicity profile of conventional XELOX and bevacizumab chemotherapy plus simvastatin in metastatic colorectal cancer patients (MCRC).
MATERIALS AND METHODS
Patients with MCRC received first-line XELOX in 3-week treatment cycles of intravenous oxaliplatin 130 mg/m² plus bevacizumab 7.5 mg/kg (day 1), followed by oral capecitabine 1,000 mg/m² twice daily (day 1-14). Simvastatin 80 mg tablets were taken orally once daily every day during the period of chemotherapy. The primary endpoint was progression-free survival (PFS). Secondary endpoints were response rate, duration of response, overall survival (OS), time to progression, and toxicity.
RESULTS
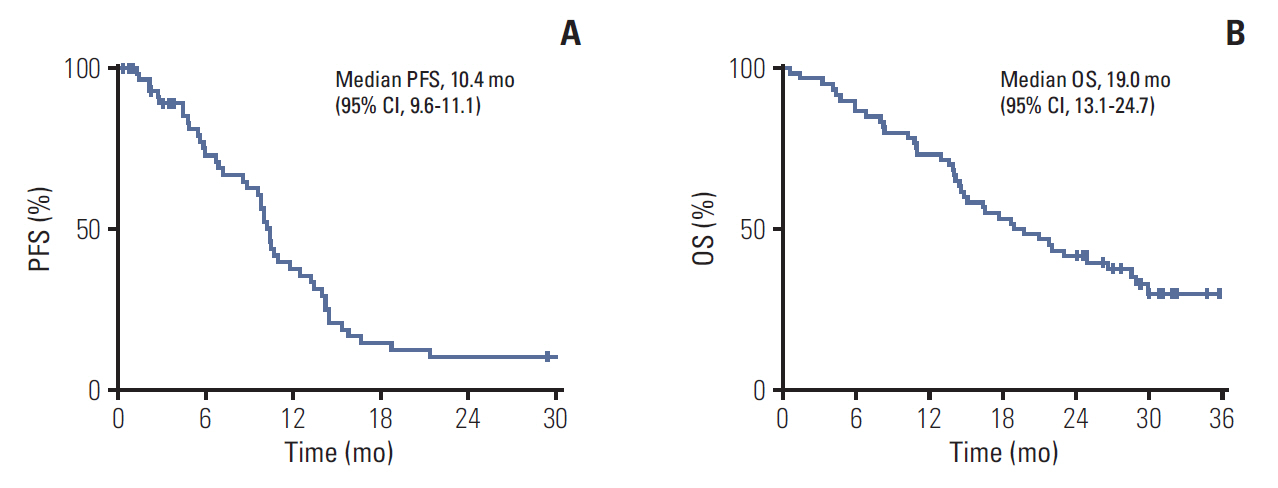
From January 2014 to April 2015, 60 patients were enrolled and 55 patients were evaluable for tumor response. The median follow-up duration was 30.1 months (range, 28.5 to 31.7 months). The median PFS was 10.4 months (95% confidence interval [CI], 9.6 to 11.1). The median OS of all patients was 19.0 months (95% CI, 11.9 to 26.0). The disease-control rate and overall response rate were 88.3% (95% CI, 74 to 96) and 58.3% (95% CI, 44 to 77), respectively, by intent-to-treat protocol analysis. There was one complete response and 34 partial responses. One patient experienced grade 3 creatine kinase elevation and liver enzyme elevation.
CONCLUSION
Based on the current study, the addition of 80 mg simvastatin to XELOX and bevacizumab showed comparable clinical efficacy in patients with MCRC as first-line chemotherapy and did not increase toxicity.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017; 67:177–93.
Article2. Matsui T, Nagata N, Hirata K, Okazaki S, Sato S, Nakamura M, et al. Bi-weekly capecitabine-oxaliplatin (XELOX) plus bevacizumab as first-line treatment of metastatic colorectal cancer: the PHOENiX Trial. Anticancer Res. 2016; 36:3437–43.3. Lee JJ, Chu E. An update on treatment advances for the first-line therapy of metastatic colorectal cancer. Cancer J. 2007; 13:276–81.
Article4. Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005; 69 Suppl 3:4–10.
Article5. Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007; 25:1539–44.
Article6. Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008; 26:5326–34.
Article7. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350:2335–42.
Article8. Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008; 26:2013–9.
Article9. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990; 343:425–30.
Article10. Osmak M. Statins and cancer: current and future prospects. Cancer Lett. 2012; 324:1–12.
Article11. Kubatka P, Kruzliak P, Rotrekl V, Jelinkova S, Mladosievicova B. Statins in oncological research: from experimental studies to clinical practice. Crit Rev Oncol Hematol. 2014; 92:296–311.
Article12. Chae YK, Yousaf M, Malecek MK, Carneiro B, Chandra S, Kaplan J, et al. Statins as anti-cancer therapy; Can we translate preclinical and epidemiologic data into clinical benefit? Discov Med. 2015; 20:413–27.13. Denoyelle C, Vasse M, Korner M, Mishal Z, Ganne F, Vannier JP, et al. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis. 2001; 22:1139–48.
Article14. Keyomarsi K, Sandoval L, Band V, Pardee AB. Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res. 1991; 51:3602–9.15. Prasanna P, Thibault A, Liu L, Samid D. Lipid metabolism as a target for brain cancer therapy: synergistic activity of lovastatin and sodium phenylacetate against human glioma cells. J Neurochem. 1996; 66:710–6.
Article16. Jakobisiak M, Bruno S, Skierski JS, Darzynkiewicz Z. Cell cycle-specific effects of lovastatin. Proc Natl Acad Sci U S A. 1991; 88:3628–32.
Article17. Lee SJ, Lee I, Lee J, Park C, Kang WK. Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, potentiate the anti-angiogenic effects of bevacizumab by suppressing angiopoietin2, BiP, and Hsp90alpha in human colorectal cancer. Br J Cancer. 2014; 111:497–505.18. Lee J, Jung KH, Park YS, Ahn JB, Shin SJ, Im SA, et al. Simvastatin plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) as first-line chemotherapy in metastatic colorectal patients: a multicenter phase II study. Cancer Chemother Pharmacol. 2009; 64:657–63.
Article19. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.20. Negre-Aminou P, van Vliet AK, van Erck M, van Thiel GC, van Leeuwen RE, Cohen LH. Inhibition of proliferation of human smooth muscle cells by various HMG-CoA reductase inhibitors; comparison with other human cell types. Biochim Biophys Acta. 1997; 1345:259–68.21. Pirillo A, Jacoviello C, Longoni C, Radaelli A, Catapano AL. Simvastatin modulates the heat shock response and cytotoxicity mediated by oxidized LDL in cultured human endothelial smooth muscle cells. Biochem Biophys Res Commun. 1997; 231:437–41.
Article22. Schaefer CA, Kuhlmann CR, Gast C, Weiterer S, Li F, Most AK, et al. Statins prevent oxidized low-density lipoprotein- and lysophosphatidylcholine-induced proliferation of human endothelial cells. Vascul Pharmacol. 2004; 41:67–73.
Article23. Alber HF, Dulak J, Frick M, Dichtl W, Schwarzacher SP, Pachinger O, et al. Atorvastatin decreases vascular endothelial growth factor in patients with coronary artery disease. J Am Coll Cardiol. 2002; 39:1951–5.24. Kabbinavar FF, Flynn PJ, Kozloff M, Ashby MA, Sing A, Barr CE, et al. Gastrointestinal perforation associated with bevacizumab use in metastatic colorectal cancer: results from a large treatment observational cohort study. Eur J Cancer. 2012; 48:1126–32.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bevacizumab induced intestinal perforation in patients with colorectal cancer
- Retrospective analysis on the clinical efficacy of bevacizumab combined with FOLFOX4 in the first line treatment of metastatic colorectal cancer
- QL1604 plus paclitaxel-cisplatin/ carboplatin in patients with recurrent or metastatic cervical cancer: an open-label, single-arm, phase II trial
- Imrpoving Outcomes with Chemotherapy in Colorectal Cancer: Current Options, Current Evidence
- Chemotherapy in Rectal Cancer