This article has been retracted.
See the retraction notice
See the retraction notice
Cancer Res Treat.
2019 Jul;51(3):1022-1032. 10.4143/crt.2018.473.
Dietary Intake of Omega-3 fatty acids and Endocrine-related Gynecological Cancer: A Meta-Analysis of Observational Studies
- Affiliations
-
- 1Department of Cancer Control and Population Health, National Cancer Center Graduate School of Cancer Science and Policy, Goyang, Korea.
- 2Department of Cancer Biomedical Science, National Cancer Center Graduate School of Cancer Science and Policy, Goyang, Korea. msk@ncc.re.kr
- 3Cancer Epidemiology Branch, National Cancer Center Research Institute, Goyang, Korea.
- 4Department of Family Medicine and Center for Cancer Prevention and Detection, National Cancer Center Hospital, Goyang, Korea.
- KMID: 2454294
- DOI: http://doi.org/10.4143/crt.2018.473
Abstract
- PURPOSE
Previous observational epidemiological studies have reported inconsistent findings on the association between dietary intake of omega-3 fatty acids and endocrine-related gynecological cancer such as ovarian cancer and endometrial cancer. This study aimed to investigate this association using a meta-analysis of observational studies.
MATERIALS AND METHODS
We searched PubMed, EMBASE, and Cochrane library by using key words related with the topic in April 2017. The pooled odd ratios (pORs), relative risks (pRRs), or hazard ratios (pHRs) with 95% confidence intervals (CIs) were calculated based on the random-effects model. Also, we performed subgroup meta-analysis by methodological quality, types of cancer, study design, and omega-3 fatty acids.
RESULTS
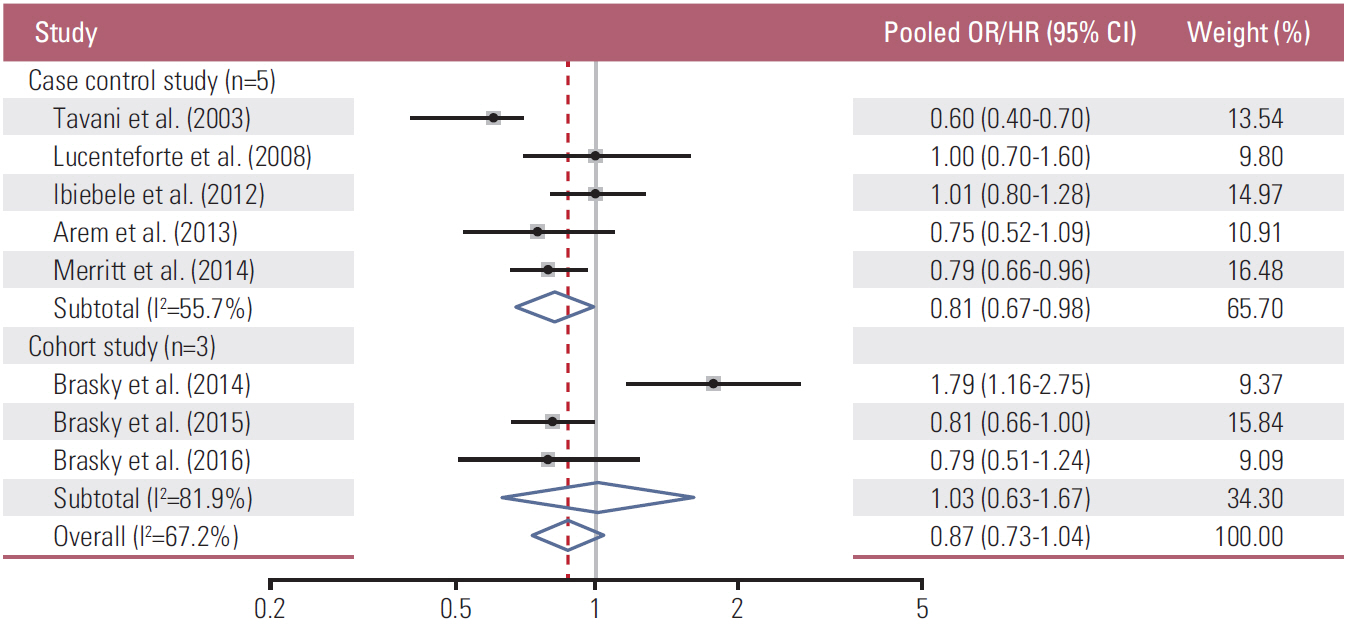
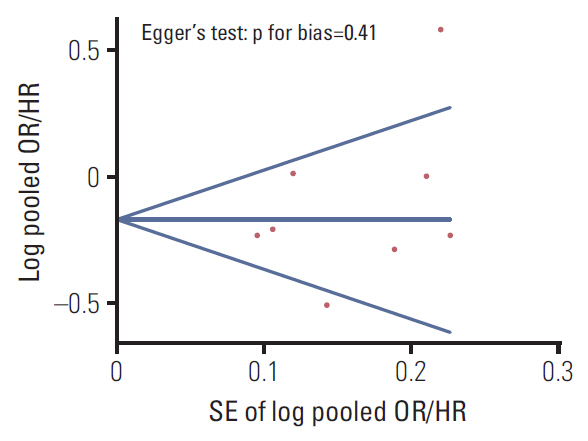
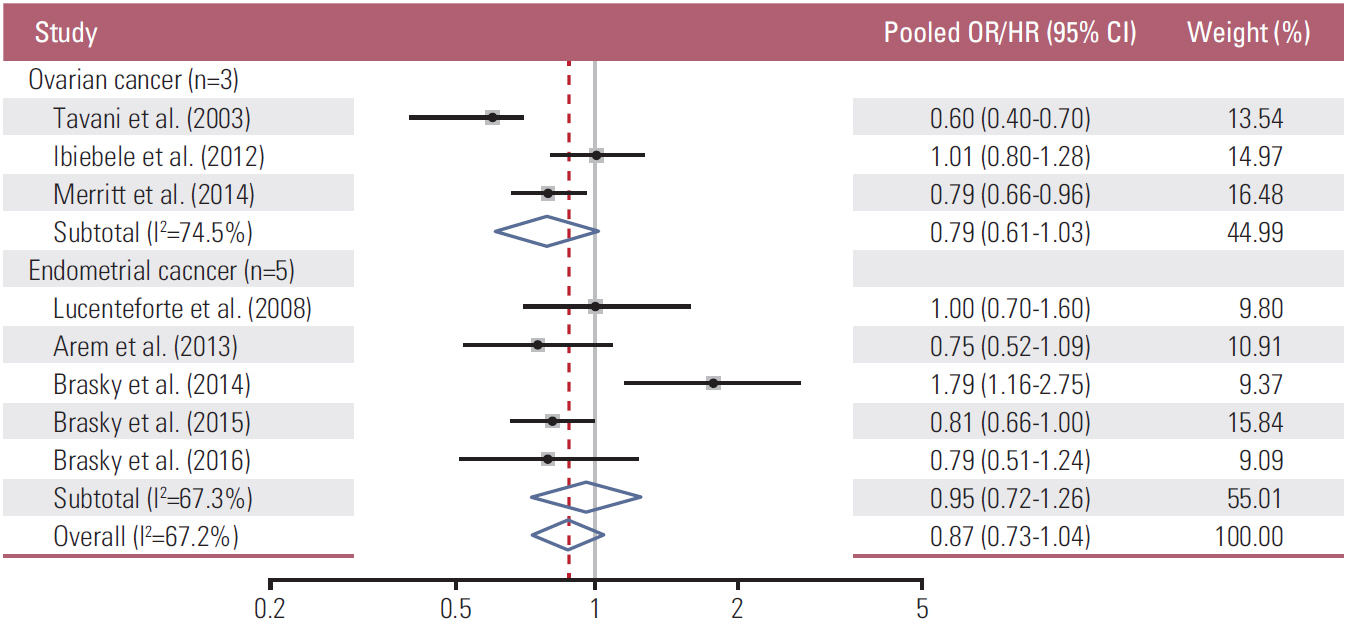
A total of ten observational studies with six case-control and four cohort studies were included in the final meta-analysis. In the meta-analysis of all the studies, dietary intake of total omega-3 fatty acids was not significantly associated with the risk of endometrial and ovarian cancers (pOR/HR, 0.87; 95% CI, 0.73-1.04; I²=67.2%) (highest versus lowest intake). In the subgroup meta-analysis by type of study, there was no significant association between them in cohort studies (pHR, 1.03; 95% CI, 0.63-1.67, I²=81.9%), whereas its reduced risk was observed in case-control studies (pOR, 0.81; 95% CI, 0.67 to 0.98, I²=55.7%).
CONCLUSION
The current meta-analysis of observational studies suggests that there is no higher level of evidence to support the protective effect of dietary omega-3 fatty acids on endocrine-related gynecological cancer. Further prospective studies should be conducted to confirm the association.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
RETRACTION: Dietary Intake of Omega-3 Fatty Acids and Endocrine-Related Gynecological Cancer: A Meta-Analysis of Observational Studies
Cancer Res Treat. 2020;52(1):334-334. doi: 10.4143/crt.2018.473.RE.
Reference
-
References
1. Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, Edwards RP, et al. Factors associated with type I and type II endometrial cancer. Cancer Causes Control. 2010; 21:1851–6.
Article2. Gibson DA, Simitsidellis I, Collins F, Saunders PT. Evidence of androgen action in endometrial and ovarian cancers. Endocr Relat Cancer. 2014; 21:T203–18.
Article3. Dossus L, Rinaldi S, Becker S, Lukanova A, Tjonneland A, Olsen A, et al. Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study. Endocr Relat Cancer. 2010; 17:1007–19.
Article4. Wang T, Rohan TE, Gunter MJ, Xue X, Wactawski-Wende J, Rajpathak SN, et al. A prospective study of inflammation markers and endometrial cancer risk in postmenopausal hormone nonusers. Cancer Epidemiol Biomarkers Prev. 2011; 20:971–7.
Article5. McSorley MA, Alberg AJ, Allen DS, Allen NE, Brinton LA, Dorgan JF, et al. C-reactive protein concentrations and subsequent ovarian cancer risk. Obstet Gynecol. 2007; 109:933–41.
Article6. Verdoodt F, Friis S, Dehlendorff C, Albieri V, Kjaer SK. Non-steroidal anti-inflammatory drug use and risk of endometrial cancer: a systematic review and meta-analysis of observational studies. Gynecol Oncol. 2016; 140:352–8.
Article7. Murphy MA, Trabert B, Yang HP, Park Y, Brinton LA, Hartge P, et al. Non-steroidal anti-inflammatory drug use and ovarian cancer risk: findings from the NIH-AARP Diet and Health Study and systematic review. Cancer Causes Control. 2012; 23:1839–52.
Article8. Kantor ED, Lampe JW, Vaughan TL, Peters U, Rehm CD, White E. Association between use of specialty dietary supplements and C-reactive protein concentrations. Am J Epidemiol. 2012; 176:1002–13.
Article9. Micallef MA, Munro IA, Garg ML. An inverse relationship between plasma n-3 fatty acids and C-reactive protein in healthy individuals. Eur J Clin Nutr. 2009; 63:1154–6.
Article10. Ebrahimi M, Ghayour-Mobarhan M, Rezaiean S, Hoseini M, Parizade SM, Farhoudi F, et al. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol. 2009; 64:321–7.11. Malekshahi Moghadam A, Saedisomeolia A, Djalali M, Djazayery A, Pooya S, Sojoudi F. Efficacy of omega-3 fatty acid supplementation on serum levels of tumour necrosis factor-alpha, C-reactive protein and interleukin-2 in type 2 diabetes mellitus patients. Singapore Med J. 2012; 53:615–9.12. Micallef MA, Garg ML. Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals. Atherosclerosis. 2009; 204:476–82.
Article13. Bertone ER, Rosner BA, Hunter DJ, Stampfer MJ, Speizer FE, Colditz GA, et al. Dietary fat intake and ovarian cancer in a cohort of US women. Am J Epidemiol. 2002; 156:22–31.
Article14. Bidoli E, La Vecchia C, Montella M, Maso LD, Conti E, Negri E, et al. Nutrient intake and ovarian cancer: an Italian case-control study. Cancer Causes Control. 2002; 13:255–61.15. Tavani A, Pelucchi C, Parpinel M, Negri E, Franceschi S, Levi F, et al. n-3 polyunsaturated fatty acid intake and cancer risk in Italy and Switzerland. Int J Cancer. 2003; 105:113–6.
Article16. Lucenteforte E, Talamini R, Montella M, Dal Maso L, Tavani A, Deandrea S, et al. Macronutrients, fatty acids and cholesterol intake and endometrial cancer. Ann Oncol. 2008; 19:168–72.
Article17. Ibiebele TI, Nagle CM, Bain CJ, Webb PM. Intake of omega-3 and omega-6 fatty acids and risk of ovarian cancer. Cancer Causes Control. 2012; 23:1775–83.
Article18. Brasky TM, Neuhouser ML, Cohn DE, White E. Associations of long-chain omega-3 fatty acids and fish intake with endometrial cancer risk in the VITamins And Lifestyle cohort. Am J Clin Nutr. 2014; 99:599–608.19. Merritt MA, Cramer DW, Missmer SA, Vitonis AF, Titus LJ, Terry KL. Dietary fat intake and risk of epithelial ovarian cancer by tumour histology. Br J Cancer. 2014; 110:1392–401.
Article20. Brasky TM, Rodabough RJ, Liu J, Kurta ML, Wise LA, Orchard TS, et al. Long-chain omega-3 fatty acid intake and endometrial cancer risk in the Women's Health Initiative. Am J Clin Nutr. 2015; 101:824–34.21. Brasky TM, Sponholtz TR, Palmer JR, Rosenberg L, Ruiz-Narvaez EA, Wise LA. Associations of dietary long-chain omega-3 polyunsaturated fatty acids and fish consumption with endometrial cancer risk in the Black women's health study. Am J Epidemiol. 2016; 183:199–209.22. Arem H, Neuhouser ML, Irwin ML, Cartmel B, Lu L, Risch H, et al. Omega-3 and omega-6 fatty acid intakes and endometrial cancer risk in a population-based case-control study. Eur J Nutr. 2013; 52:1251–60.
Article23. Wells GA, Shea B, Connell DO, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa: Ottawa Hospital Research Institute;2000. [cited 2017 Sep 18]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.24. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–58.
Article25. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions [Internet]. London: Cochrane Collaboration;2011. [cited 2017 Sep 18]. Available from: http://www.handbook.cochrane.org/.26. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010; 1:97–111.
Article27. Choi YJ, Myung SK, Lee JH. Light alcohol drinking and risk of cancer: a meta-analysis of cohort studies. Cancer Res Treat. 2018; 50:474–87.
Article28. Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001; 20:641–54.
Article29. Hammer GP, du Prel JB, Blettner M. Avoiding bias in observational studies: part 8 in a series of articles on evaluation of scientific publications. Dtsch Arztebl Int. 2009; 106:664–8.30. Giovannucci E, Stampfer MJ, Colditz GA, Manson JE, Rosner BA, Longnecker M, et al. A comparison of prospective and retrospective assessments of diet in the study of breast cancer. Am J Epidemiol. 1993; 137:502–11.
Article31. Qiu W, Lu H, Qi Y, Wang X. Dietary fat intake and ovarian cancer risk: a meta-analysis of epidemiological studies. Oncotarget. 2016; 7:37390–406.
Article32. Zhao J, Lyu C, Gao J, Du L, Shan B, Zhang H, et al. Dietary fat intake and endometrial cancer risk: a dose response meta-analysis. Medicine (Baltimore). 2016; 95:e4121.33. Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009; 81:187–91.
Article34. Zheng H, Tang H, Liu M, He M, Lai P, Dong H, et al. Inhibition of endometrial cancer by n-3 polyunsaturated fatty acids in preclinical models. Cancer Prev Res (Phila). 2014; 7:824–34.
Article35. Sharma A, Belna J, Logan J, Espat J, Hurteau JA. The effects of omega-3 fatty acids on growth regulation of epithelial ovarian cancer cell lines. Gynecol Oncol. 2005; 99:58–64.
Article36. Wan XH, Fu X, Ababaikeli G. Docosahexaenoic acid induces growth suppression on epithelial ovarian cancer cells more effectively than eicosapentaenoic acid. Nutr Cancer. 2016; 68:320–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RETRACTION: Dietary Intake of Omega-3 Fatty Acids and Endocrine-Related Gynecological Cancer: A Meta-Analysis of Observational Studies
- Association between dietary omega-3 fatty acid intake and depression in postmenopausal women
- Polyunsaturated Fatty Acids in Children
- Dietary intake of fat and fatty acids by 1–5-year-old children in Korea: a cross-sectional study based on data from the sixth Korea National Health and Nutrition Examination Survey
- Omega-3 Index as a Risk Factor for Cardiovascular Disease and Its Application to Korean Population