Nutr Res Pract.
2019 Aug;13(4):302-309. 10.4162/nrp.2019.13.4.302.
Carpinus turczaninowii extract modulates arterial inflammatory response: a potential therapeutic use for atherosclerosis
- Affiliations
-
- 1Biological and Genetic Resources Assessment Division, National Institute of Biological Resources, Incheon 22689, Republic of Korea.
- 2Department of Food Science and Nutrition, Dong-A University, Busan 49315, Republic of Korea. oykim@dau.ac.kr
- 3Center for Silver-targeted Biomaterials, Brain Busan 21 Plus Program, Dong-A University, 37 Nakdongdae-ro, 550beon-gil Saha-gu, Busan, 49315, Republic of Korea.
- 4Institute of Health Insurance and Clinical Research, National Health Insurance Service Ilsan Hospital, Gyeonggi, 10444, Republic of Korea.
- 5Departments of Anatomy, Chonnam National University Medical School, Gwangju, 61469, Republic of Korea.
- KMID: 2453108
- DOI: http://doi.org/10.4162/nrp.2019.13.4.302
Abstract
- BACKGROUND/OBJECTIVES
Vascular inflammation is an important feature in the atherosclerotic process. Recent studies report that leaves and branches of Carpinus turczaninowii (C. turczaninowii) have antioxidant capacity and exert anti-inflammatory effects. However, no study has reported the regulatory effect of C. turczaninowii extract on the arterial inflammatory response. This study therefore investigated modulation of the arterial inflammatory response after exposure to C. turczaninowii extract, using human aortic vascular smooth muscle cells (HAoSMCs).
MATERIALS/METHODS
Scavenging activity of free radicals, total phenolic content (TPC), cell viability, mRNA expressions, and secreted levels of cytokines were measured in LPS-stimulated (10 ng/mL) HAoSMCs treated with the C. turczaninowii extract.
RESULTS
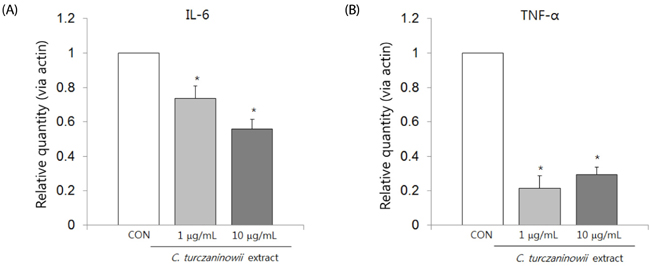
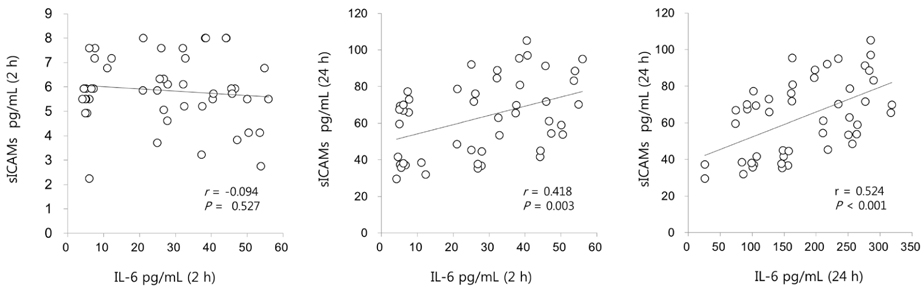
C. turczaninowii extract contains high amounts of TPC (225.6 ± 21.0 mg of gallic acid equivalents/g of the extract), as well as exerts time-and dose-dependent increases in strongly scavenged free radicals (average 14.8 ± 1.97 µg/mL IC50 at 40 min). Cell viabilities after exposure to the extracts (1 and 10 µg/mL) were similar to the viability of non-treated cells. Cytokine mRNA expressions were significantly suppressed by the extracts (1 and 10 µg/mL) at 6 hours (h) after exposure. Interleukin-6 secretion was dose-dependently suppressed 2 h after incubation with the extract, at 1-10 µg/mL in non-stimulated cells, and at 5 and 10 µg/mL in LPS-stimulated cells. Similar patterns were also observed at 24 h after incubation with the extract (at 1-10 µg/mL in non-stimulated cells, and at 10 µg/mL in the LPS-stimulated cells). Soluble intracellular vascular adhesion molecules (sICAM-1) secreted from non-stimulated cells and LPS-stimulated cells were similarly suppressed in a dose-dependent manner after 24 h exposure to the extracts, but not after 2 h. In addition, sICAM-1 concentration after 24 h treatment was positively related to IL-6 levels after 2 h and 24 h exposure (r = 0.418, P = 0.003, and r = 0.524, P < 0.001, respectively).
CONCLUSIONS
This study demonstrates that C. turczaninowii modulates the arterial inflammatory response, and indicates the potential to be applied as a therapeutic use for atherosclerosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011; 12:204–212.
Article2. Wright E Jr, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract. 2006; 60:308–314.
Article3. Ohnuki Y, Nagano R, Takizawa S, Takagi S, Miyata T. Advanced glycation end products in patients with cerebral infarction. Intern Med. 2009; 48:587–591.
Article4. Lönn ME, Dennis JM, Stocker R. Actions of “antioxidants” in the protection against atherosclerosis. Free Radic Biol Med. 2012; 53:863–884.
Article5. Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003; 52:2795–2804.
Article6. Chae JS, Kim OY, Paik JK, Kang R, Seo WJ, Jeong TS, Sweeney G, Lee SH, Lee JH. Association of Lp-PLA(2) activity and LDL size with interleukin-6, an inflammatory cytokine and oxidized LDL, a marker of oxidative stress, in women with metabolic syndrome. Atherosclerosis. 2011; 218:499–506.
Article7. Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012; 33:829–837.
Article8. Halford JC. Obesity drugs in clinical development. Curr Opin Investig Drugs. 2006; 7:312–318.9. Marazza JA, Nazareno MA, de Giori GS, Garro MS. Enhancement of the antioxidant capacity of soymilk by fermentation with Lactobacillus rhamnosus. J Funct Foods. 2012; 4:594–601.
Article10. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993; 342:1007–1011.
Article11. Chen Y, Wang J, Ou Y, Chen H, Xiao S, Liu G, Cao Y, Huang Q. Cellular antioxidant activities of polyphenols isolated from Eucalyptus leaves (Eucalyptus grandis × Eucalyptus urophylla GL9). J Funct Foods. 2014; 7:737–745.
Article12. Zheng G, Xu L, Wu P, Xie H, Jiang Y, Chen F, Wei X. Polyphenols from longan seeds and their radical-scavenging activity. Food Chem. 2009; 116:433–436.
Article13. Jin T, Kim OY, Shin MJ, Choi EY, Lee SS, Han YS, Chung JH. Fisetin up-regulates the expression of adiponectin in 3T3-L1 adipocytes via the activation of silent mating type information regulation 2 homologue 1 (SIRT1)-deacetylase and peroxisome proliferator-activated receptors (PPARs). J Agric Food Chem. 2014; 62:10468–10474.
Article14. Moon J, Do HJ, Kim OY, Shin MJ. Antiobesity effects of quercetin-rich onion peel extract on the differentiation of 3T3-L1 preadipocytes and the adipogenesis in high fat-fed rats. Food Chem Toxicol. 2013; 58:347–354.
Article15. Ko HN, Oh TH, Baik JS, Hyun CG, Kim SS, Lee NH. Anti-inflammatory activities for the extracts and Carpinontriols from branches of Caripinus Turczaninowii. Int J Pharmacol. 2013; 9:157–163.
Article16. Ko HN, Kim JM, Bu HJ, Lee NH. Chemical constituents from the branches of Carpinus turczaninowii with antioxidative activities. J Korean Chem Soc. 2013; 57:520–524.
Article17. Kang JM. Identification of anti-oxidative, skin whitening, anti-inflammatory and anti-bacterial constituents from the leaves of Carpinus turczaninowii Hance [master's thesis]. Jeju: Jeju National University;2015.18. Jeon JI, Chang CS. Foliar flavonoids of genus Carpinus in eastern Asia primarily based on native taxa to Korea. Korean J Plant Taxon. 2000; 30:139–153.
Article19. Chang CS, Chang KS. Typification of Corylopsis coreana (Hamamelidaceae) and Carpinus laxiflora var. longispica (Betulaceae). Shokubutsu Kenkyu Zasshi. 2010; 85:270–276.20. Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995; 28:25–30.21. Deshpande SS, Cheryan M. Determination of phenolic compounds of dry beans using vanillin, redox and precipitation assays. J Food Sci. 1987; 52:332–334.
Article22. Yang EJ, Yim EY, Song G, Kim GO, Hyun CG. Inhibition of nitric oxide production in lipopolysaccharide-activated RAW 264.7 macrophages by Jeju plant extracts. Interdiscip Toxicol. 2009; 2:245–249.
Article23. Libby P, Ridker PM. Novel inflammatory markers of coronary risk: theory versus practice. Circulation. 1999; 100:1148–1150.24. Schildberger A, Rossmanith E, Eichhorn T, Strassl K, Weber V. Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediators Inflamm. 2013; 2013:697972.
Article25. Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010; 86:211–218.
Article26. Rothlein R, Mainolfi EA, Czajkowski M, Marlin SD. A form of circulating ICAM-1 in human serum. J Immunol. 1991; 147:3788–3793.27. Rieckmann P, Michel U, Albrecht M, Brück W, Wöckel L, Felgenhauer K. Soluble forms of intercellular adhesion molecule-1 (ICAM-1) block lymphocyte attachment to cerebral endothelial cells. J Neuroimmunol. 1995; 60:9–15.
Article28. Vejlsgaard GL, Ralfkiaer E, Avnstorp C, Czajkowski M, Marlin SD, Rothlein R. Kinetics and characterization of intercellular adhesion molecule-1 (ICAM-1) expression on keratinocytes in various inflammatory skin lesions and malignant cutaneous lymphomas. J Am Acad Dermatol. 1989; 20:782–790.
Article29. Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989; 83:2008–2017.
Article30. Shijubo N, Imai K, Shigehara K, Honda Y, Koba H, Tsujisaki M, Hinoda Y, Yachi A, Ohmichi M, Hiraga Y, Abe S. Soluble intercellular adhesion molecule-1 (ICAM-1) in sera and bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Clin Exp Immunol. 1994; 95:156–161.
Article31. Tsukada N, Matsuda M, Miyagi K, Yanagisawa N. Increased levels of intercellular adhesion molecule-1 (ICAM-1) and tumor necrosis factor receptor in the cerebrospinal fluid of patients with multiple sclerosis. Neurology. 1993; 43:2679–2682.
Article32. Teppo AM, von Willebrand E, Honkanen E, Ahonen J, Grönhagen-Riska C. Soluble intercellular adhesion molecule-1 (sICAM-1) after kidney transplantation: the origin and role of urinary sICAM-1? Transplantation. 2001; 71:1113–1119.
Article33. Bongard V, Elias A, Bal dit Sollier C, Ruidavets J, Boccalon H, Drouet L, Ferrières J. Soluble intercellular adhesion molecule-1 is associated with carotid and femoral atherosclerosis but not with intima-media thickness in a population-based sample. Atherosclerosis. 2002; 164:297–304.
Article34. Blann AD, Steele C, McCollum CN. The influence of smoking on soluble adhesion molecules and endothelial cell markers. Thromb Res. 1997; 85:433–438.
Article35. Rohde LE, Hennekens CH, Ridker PM. Cross-sectional study of soluble intercellular adhesion molecule-1 and cardiovascular risk factors in apparently healthy men. Arterioscler Thromb Vasc Biol. 1999; 19:1595–1599.
Article36. Hackman A, Abe Y, Insull W Jr, Pownall H, Smith L, Dunn K, Gotto AM Jr, Ballantyne CM. Levels of soluble cell adhesion molecules in patients with dyslipidemia. Circulation. 1996; 93:1334–1338.
Article37. Abe Y, El-Masri B, Kimball KT, Pownall H, Reilly CF, Osmundsen K, Smith CW, Ballantyne CM. Soluble cell adhesion molecules in hypertriglyceridemia and potential significance on monocyte adhesion. Arterioscler Thromb Vasc Biol. 1998; 18:723–731.
Article38. Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998; 351:88–92.
Article39. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999; 340:115–126.40. Marino M, Scuderi F, Mazzarelli P, Mannella F, Provenzano C, Bartoccioni E. Constitutive and cytokine-induced expression of MHC and intercellular adhesion molecule-1 (ICAM-1) on human myoblasts. J Neuroimmunol. 2001; 116:94–101.
Article41. Ghaisas NK, Shahi CN, Foley B, Goggins M, Crean P, Kelly A, Kelleher D, Walsh M. Elevated levels of circulating soluble adhesion molecules in peripheral blood of patients with unstable angina. Am J Cardiol. 1997; 80:617–619.
Article42. Frohman EM, Frohman TC, Dustin ML, Vayuvegula B, Choi B, Gupta A, van den Noort S, Gupta S. The induction of intercellular adhesion molecule 1 (ICAM-1) expression on human fetal astrocytes by interferon-gamma, tumor necrosis factor alpha, lymphotoxin, and interleukin-1: relevance to intracerebral antigen presentation. J Neuroimmunol. 1989; 23:117–124.
Article43. Thomson AW, Satoh S, Nüssler AK, Tamura K, Woo J, Gavaler J, van Thiel DH. Circulating intercellular adhesion molecule-1 (ICAM-1) in autoimmune liver disease and evidence for the production of ICAM-1 by cytokine-stimulated human hepatocytes. Clin Exp Immunol. 1994; 95:83–90.
Article44. Chen NG, Azhar S, Abbasi F, Carantoni M, Reaven GM. The relationship between plasma glucose and insulin responses to oral glucose, LDL oxidation, and soluble intercellular adhesion molecule-1 in healthy volunteers. Atherosclerosis. 2000; 152:203–208.
Article45. Aljada A, Saadeh R, Assian E, Ghanim H, Dandona P. Insulin inhibits the expression of intercellular adhesion molecule-1 by human aortic endothelial cells through stimulation of nitric oxide. J Clin Endocrinol Metab. 2000; 85:2572–2575.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Chloroform Fraction of Carpinus tschonoskii Leaves Inhibits the Production of Inflammatory Mediators in HaCaT Keratinocytes and RAW264.7 Macrophages
- Anti-inflammatory Activity of Carpinus tschonoskii Leaves Extract in R848-stimulated Bone Marrow-derived Macrophages and Dendritic Cells
- Effect of Ginseng Extract on Blood Lipids and Atherosclerosis
- Therapeutic Potential of Methanol Extract of Euonymus alatus in HT22 Cells Through Neuroprotective Mechanisms
- Role of Nucleotide-binding and Oligomerization Domain 2 Protein (NOD2) in the Development of Atherosclerosis