Nat Prod Sci.
2019 Jun;25(2):103-110. 10.20307/nps.2019.25.2.103.
Effect of Pyunkang-tang on Inflammatory Aspects of Chronic Obstructive Pulmonary Disease in a Rat Model
- Affiliations
-
- 1Department of Pharmacology, School of Medicine, Chungnam National University, Daejeon, Korea. LCJ123@cnu.ac.kr
- 2Smith Liberal Arts College and Department of Addiction Science, Graduate School, Sahmyook University, Seoul, Korea.
- KMID: 2452778
- DOI: http://doi.org/10.20307/nps.2019.25.2.103
Abstract
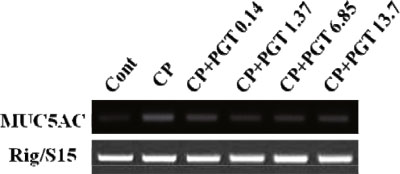
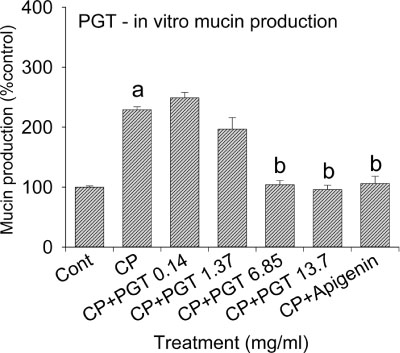
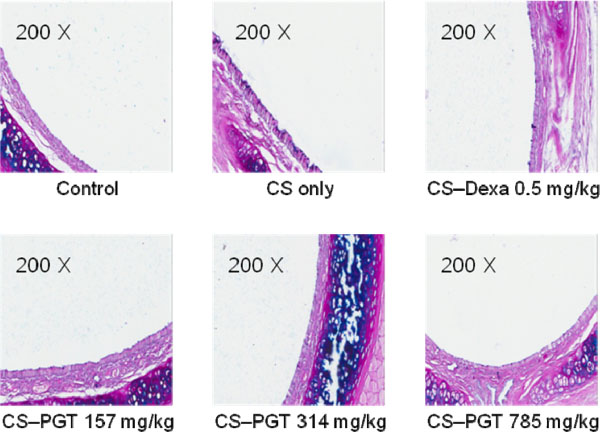
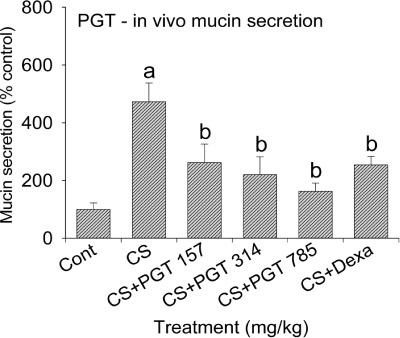
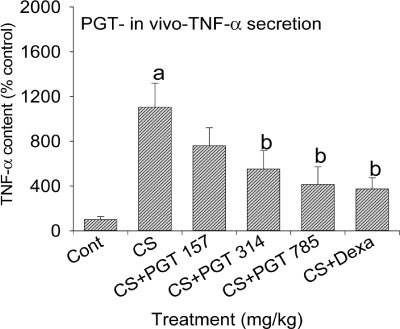
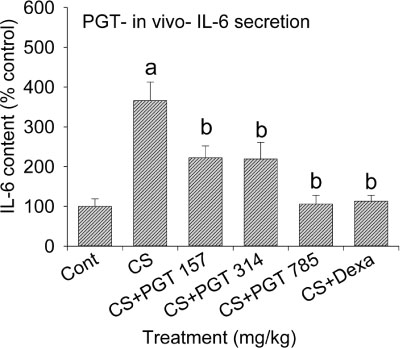
- We investigated the anti-inflammatory effect of Pyunkang-tang extract (PGT), a complex herbal extract based on traditional Chinese medicine that is used in Korea for controlling diverse pulmonary diseases, on cigarette smoke-induced pulmonary pathology in a rat model of chronic obstructive pulmonary disease (COPD). The constituents of PGT were Lonicerae japonica, Liriope platyphylla, Adenophora triphilla, Xantium strumarinum, Selaginella tamariscina and Rehmannia glutinosa. Rats were exposed by inhalation to a mixture of cigarette smoke extract (CSE) and sulfur dioxide for three weeks to induce COPD-like pulmonary inflammation. PGT was administered orally to rats and pathological changes to the pulmonary system were examined in each group of animals through measurement of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) levels in bronchoalveolar lavage fluid (BALF) at 21 days post-CSE treatment. The effect of PGT on the hypersecretion of pulmonary mucin in rats was assessed by quantification of the amount of mucus secreted and by examining histopathologic changes in tracheal epithelium. Confluent NCI-H292 cells were pretreated with PGT for 30 min and then stimulated with CSE plus PMA (phorbol 12-myristate 13-acetate), for 24 h. The MUC5AC mucin gene expression was measured by RT-PCR. Production of MUC5AC mucin protein was measured by ELISA. The results were as follows: (1) PGT inhibited CSE-induced pulmonary inflammation as shown by decreased TNF-α and IL-6 levels in BALF; (2) PGT inhibited the hypersecretion of pulmonary mucin and normalized the increased amount of mucosubstances in goblet cells of the CSE-induced COPD rat model; (3) PGT inhibited CSE-induced MUC5AC mucin production and gene expression in vitro in NCI-H292 cells, a human airway epithelial cell line. These results suggest that PGT might regulate the inflammatory aspects of COPD in a rat model.
Keyword
MeSH Terms
-
Animals
Bronchoalveolar Lavage Fluid
Campanulaceae
Enzyme-Linked Immunosorbent Assay
Epithelial Cells
Epithelium
Gene Expression
Goblet Cells
Humans
In Vitro Techniques
Inflammation
Inhalation
Interleukin-6
Korea
Lonicera
Lung Diseases
Medicine, Chinese Traditional
Models, Animal*
Mucins
Mucus
Necrosis
Pathology
Pneumonia
Pulmonary Disease, Chronic Obstructive*
Rats*
Rehmannia
Selaginellaceae
Smoke
Sulfur Dioxide
Tobacco Products
Interleukin-6
Mucins
Smoke
Sulfur Dioxide
Figure
Reference
-
1. Pauwels RA, Rabe KF. Lancet. 2004; 364:613–620.2. Mannino DM, Buist AS. Lancet. 2007; 370:765–773.3. Ramos FL, Krahnke JS, Kim V. Int J Chron Obstruct Pulmon Dis. 2014; 9:139–150.4. Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino D. M Eur Respir J. 2006; 28:523–532.5. Stang P, Lydick E, Silberman C, Kempel A, Keating ET. Chest. 2000; 117:354S–359S.6. Harber P, Tashkin DP, Simmons M, Crawford L, Hnizdo E, Connett J. Lung Health Study Group. Am J Respir Crit Care Med. 2007; 176:994–1000.7. Calverley PM, Walker P. Lancet. 2003; 362:1053–1061.8. Corrigan CJ, Kay AB. Am Rev Respir Dis. 1991; 143:1165–1168.9. Antoniu SA. Int J Chron Obstruct Pulmon Dis. 2011; 6:147–155.10. Siafakas NM, Vermeire P, Pride NB, Paoletti P, Gibson J, Howard P, Yernault JC, Decrame M, Higenbottam T, Postma DS, Rees J. Eur Respir J. 1995; 8:1398–1420.11. George J, Loannides-Demos LL, Santamaria NM, Kong DC, Stewart K. Med J Aust. 2004; 181:248–251.12. Shinozuka N, Tatsumi K, Nakamura A, Terada J, Kuriyama T. J Am Geriatr Soc. 2007; 55:313–314.13. Seo HS, Lee HJ, Lee CJ. J Tradit Chin Med. 2016; 36:663–670.14. Churg A, Cosio M, Wright JL. Am J Physiol Lung Cell Mol Physiol. 2008; 294:L612–L631.15. Cuzić S, Bosnar M, Kramarić MD, Ferencić Z, Marković D, Glojnarić I, Eraković Haber V. Toxicol Pathol. 2012; 40:1169–1187.16. O'Donnell RA, Peebles C, Ward JA, Daraker A, Angco G, Broberg P, Pierrou S, Lund J, Holgate ST, Davies DE, Delany DJ, Wilson SJ, Djukanovic R. Thorax. 2004; 59:837–842.17. Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Int J Environ Res Public Health. 2011; 8:613–628.18. Husgafvel-Pursiainen K. Mutat Res. 2004; 567:427–445.19. Su Y, Han W, Giraldo C, De Li Y, Block ER. Am J Respir Cell Mol Biol. 1998; 19:819–825.20. Evans CM, Koo JS. Pharmacol Ther. 2009; 121:332–348.21. Rogers DF, Barnes PJ. Ann Med. 2006; 38:116–125.22. Kim KD, Lee HJ, Lim SP, Sikder A, Lee SY, Lee CJ. Phytother Res. 2012; 26:1301–1307.23. Hurst JR, Donaldson GC, Perera WR, Wilkinson TM, Bilello JA, Hagan GW, Vessey RS, Wedzicha JA. Am J Respir Crit Care Med. 2006; 174:867–874.24. Bradford E, Jacobson S, Varasteh J, Comellas AP, Woodruff P, O'Neal W, DeMeo DL, Li X, Kim V, Cho M, Castaldi PJ, Hersh C, Silverman EK, Crapo JD, Kechris K, Bowler RP. Respir Res. 2017; 18:180.25. Pant S, Walters EH, Griffiths A, Wood-Baker R, Johns DP, Reid DW. Respirology. 2009; 14:495–503.26. Lin X, Fan Y, Wang X, Chi M, Li X, Zhang X, Sun D. Am J Med Sci. 2017; 354:388–394.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cor Pulmonale with Particular Reference to Chronic Obstructive Pulmonary Disease and Pulmonary Tuberculosis
- The Effect of Respiratory Muscle Training in Patients with Chronic Obstructive Pulmonary Disease: Preliminary Study
- The Role of Innate and Adaptive Immune Cells in the Immunopathogenesis of Chronic Obstructive Pulmonary Disease
- Chronic Obstructive Pulmonary Disease: Respiratory Review of 2013
- Management of Chronic Obstructive Pulmonary Disease