Allergy Asthma Immunol Res.
2019 Sep;11(5):632-643. 10.4168/aair.2019.11.5.632.
Short-term Haze Exposure Predisposes Healthy Volunteers to Nasal Inflammation
- Affiliations
-
- 1Beijing Institute of Otolaryngology, Department of Otolaryngology Head and Neck Surgery, Beijing TongRen Hospital, Capital Medical University, Beijing, China. dr.luozhang@139.com, wangcs830@126.com
- 2Beijing Key Laboratory of Nasal Diseases, Beijing Institute of Otolaryngology, Beijing, China.
- KMID: 2452754
- DOI: http://doi.org/10.4168/aair.2019.11.5.632
Abstract
- PURPOSE
This study aimed to investigate the impact of short-term haze exposure on nasal inflammation in healthy volunteers.
METHODS
Thirty-three healthy university students were assessed for nasal symptoms, nasal patency, upper and lower respiratory tract nitric oxide (NO) as well as inflammatory mediators and neuropeptides in nasal secretions before and after a 5-day haze episode. Peripheral blood mononuclear cells (PBMCs) were stimulated with particulate matter with an aerodynamic diameter of less than 2.5 μm (PM(2.5)), and cytokines in the supernatants were examined.
RESULTS
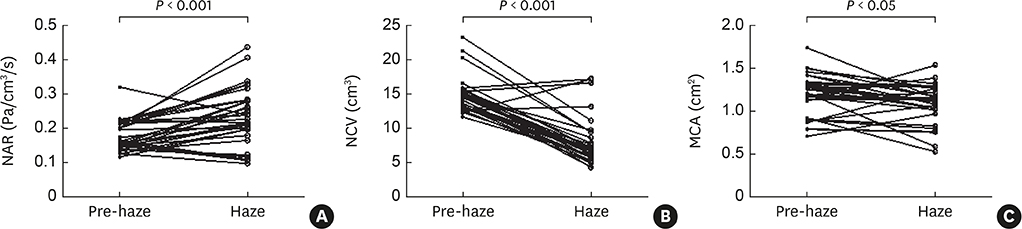
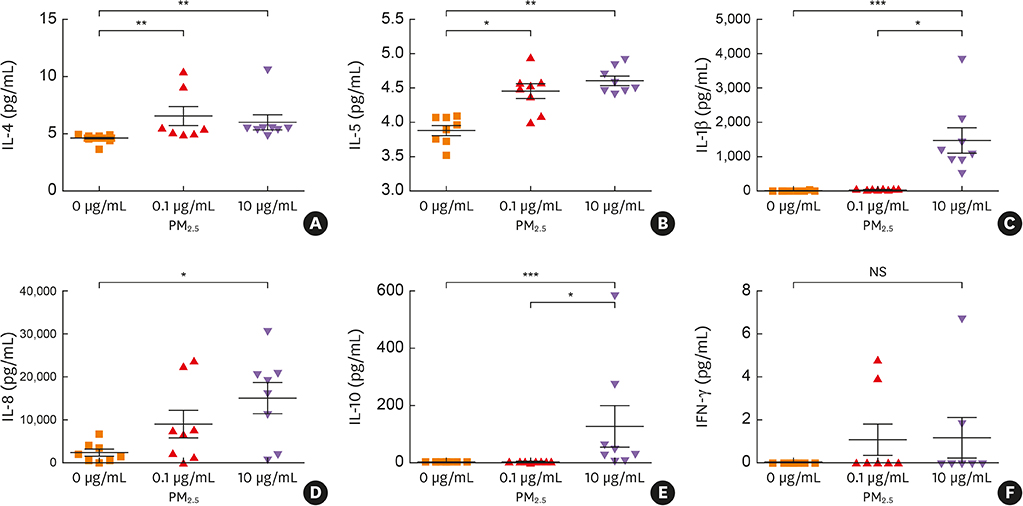
Mild nasal symptoms were reported by some participants during the haze episode. Objective measures of nasal patency demonstrated that nasal airway resistance was significantly increased from baseline levels, while nasal cavity volume and minimum cross-sectional area were significantly decreased. Similarly, the levels of nasal and exhaled NO, eotaxin, interleukin (IL)-5, chemokine (C-C motif) ligand 17, IL-8, substance P, nerve growth factor and vasoactive intestinal peptides in nasal secretions were significantly increased from baseline values following the haze episode. In contrast, the levels of interferon-γ, IL-10, transforming growth factor-β and neuropeptide Y were significantly decreased. Incubation with 0.1-10 μg/mL PM(2.5) significantly increased release of IL-1β, IL-4, IL-5, IL-8 and IL-10 from PBMCs.
CONCLUSIONS
Short-term haze exposure may lead to nasal inflammation and hypersensitivity in healthy subjects predominantly by Th2 cytokine-mediated immune responses.
MeSH Terms
-
Air Pollution
Airway Resistance
Cytokines
Healthy Volunteers*
Humans
Hypersensitivity
Inflammation*
Interleukin-10
Interleukin-4
Interleukin-5
Interleukin-8
Interleukins
Nasal Cavity
Nerve Growth Factor
Neuropeptide Y
Neuropeptides
Nitric Oxide
Particulate Matter
Peptides
Respiratory System
Substance P
Cytokines
Interleukin-10
Interleukin-4
Interleukin-5
Interleukin-8
Interleukins
Nerve Growth Factor
Neuropeptide Y
Neuropeptides
Nitric Oxide
Particulate Matter
Peptides
Substance P
Figure
Reference
-
1. Xu P, Chen Y, Ye X. Haze, air pollution, and health in China. Lancet. 2013; 382:2067.
Article2. Jin Q, Fang X, Wen B, Shan A. Spatio-temporal variations of PM2.5 emission in China from 2005 to 2014. Chemosphere. 2017; 183:429–436.
Article3. Li M, Zhang L. Haze in China: current and future challenges. Environ Pollut. 2014; 189:85–86.
Article4. Guan WJ, Zheng XY, Chung KF, Zhong NS. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016; 388:1939–1951.
Article5. Keleş N, Ilicali C. The impact of outdoor pollution on upper respiratory diseases. Rhinology. 1998; 36:24–27.6. Zhang Y, Zhang L. Prevalence of allergic rhinitis in china. Allergy Asthma Immunol Res. 2014; 6:105–113.
Article7. Chen F, Lin Z, Chen R, Norback D, Liu C, Kan H, et al. The effects of PM2.5 on asthmatic and allergic diseases or symptoms in preschool children of six Chinese cities, based on China, children, homes and health (CCHH) project. Environ Pollut. 2018; 232:329–337.
Article8. Stevens WW, Lee RJ, Schleimer RP, Cohen NA. Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol. 2015; 136:1442–1453.
Article9. Ramanathan M Jr, London NR Jr, Tharakan A, Surya N, Sussan TE, Rao X, et al. Airborne particulate matter induces nonallergic eosinophilic sinonasal inflammation in mice. Am J Respir Cell Mol Biol. 2017; 57:59–65.
Article10. Chen Y, Wong GW, Li J. Environmental exposure and genetic predisposition as risk factors for asthma in China. Allergy Asthma Immunol Res. 2016; 8:92–100.
Article11. Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014; 383:1581–1592.
Article12. Ni L, Chuang CC, Zuo L. Fine particulate matter in acute exacerbation of COPD. Front Physiol. 2015; 6:294.
Article13. Krämer U, Koch T, Ranft U, Ring J, Behrendt H. Traffic-related air pollution is associated with atopy in children living in urban areas. Epidemiology. 2000; 11:64–70.
Article14. Watelet JB, Gevaert P, Holtappels G, Van Cauwenberge P, Bachert C. Collection of nasal secretions for immunological analysis. Eur Arch Otorhinolaryngol. 2004; 261:242–246.
Article15. World Health Organization, Regional Office for Europe. Air quality guidelines global update 2005: particulate matter, ozone, nitrogen dioxide and sulfur dioxide. Copenhagen: WHO Regional Office for Europe;2006.16. Inoue Y, Sato S, Manabe T, Makita E, Chiyotanda M, Takahashi K, et al. Measurement of exhaled nitric oxide in children: a comparison between NObreath® and NIOX VERO® analyzers. Allergy Asthma Immunol Res. 2018; 10:478–489.
Article17. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.18. Scarpa MC, Kulkarni N, Maestrelli P. The role of non-invasive biomarkers in detecting acute respiratory effects of traffic-related air pollution. Clin Exp Allergy. 2014; 44:1100–1118.
Article19. Nightingale JA, Rogers DF, Barnes PJ. Effect of inhaled ozone on exhaled nitric oxide, pulmonary function, and induced sputum in normal and asthmatic subjects. Thorax. 1999; 54:1061–1069.
Article20. Olin AC, Stenfors N, Torén K, Blomberg A, Helleday R, Ledin MC, et al. Nitric oxide (NO) in exhaled air after experimental ozone exposure in humans. Respir Med. 2001; 95:491–495.
Article21. Biewenga J, Stoop AE, Baker HE, Swart SJ, Nauta JJ, van Kamp GJ, et al. Nasal secretions from patients with polyps and healthy individuals, collected with a new aspiration system: evaluation of total protein and immunoglobulin concentrations. Ann Clin Biochem. 1991; 28:260–266.
Article22. Steerenberg PA, Nierkens S, Fischer PH, van Loveren H, Opperhuizen A, Vos JG, et al. Traffic-related air pollution affects peak expiratory flow, exhaled nitric oxide, and inflammatory nasal markers. Arch Environ Health. 2001; 56:167–174.
Article23. Barraza-Villarreal A, Sunyer J, Hernandez-Cadena L, Escamilla-Nuñez MC, Sienra-Monge JJ, Ramírez-Aguilar M, et al. Air pollution, airway inflammation, and lung function in a cohort study of Mexico City schoolchildren. Environ Health Perspect. 2008; 116:832–838.
Article24. Romieu I, Barraza-Villarreal A, Escamilla-Nuñez C, Almstrand AC, Diaz-Sanchez D, Sly PD, et al. Exhaled breath malondialdehyde as a marker of effect of exposure to air pollution in children with asthma. J Allergy Clin Immunol. 2008; 121:903–909.e6.
Article25. Wu W, Peden DB, McConnell R, Fruin S, Diaz-Sanchez D. Glutathione-S-transferase M1 regulation of diesel exhaust particle-induced pro-inflammatory mediator expression in normal human bronchial epithelial cells. Part Fibre Toxicol. 2012; 9:31.
Article26. Bernstein DI. Diesel exhaust exposure, wheezing and sneezing. Allergy Asthma Immunol Res. 2012; 4:178–183.
Article27. Smarr CB, Bryce PJ, Miller SD. Antigen-specific tolerance in immunotherapy of Th2-associated allergic diseases. Crit Rev Immunol. 2013; 33:389–414.
Article28. Wawrzyniak M, O'Mahony L, Akdis M. Role of regulatory cells in oral tolerance. Allergy Asthma Immunol Res. 2017; 9:107–115.
Article29. Baraniuk JN, Lundgren JD, Okayama M, Goff J, Mullol J, Merida M, et al. Substance P and neurokinin A in human nasal mucosa. Am J Respir Cell Mol Biol. 1991; 4:228–236.
Article30. Hanf G, Schierhorn K, Brunnée T, Noga O, Verges D, Kunkel G. Substance P induced histamine release from nasal mucosa of subjects with and without allergic rhinitis. Inflamm Res. 2000; 49:520–523.
Article31. Kaliner MA. The physiology and pathophysiology of the parasympathetic nervous system in nasal disease: an overview. J Allergy Clin Immunol. 1992; 90:1044–1045.
Article32. Kim DH, Park IH, Cho JS, Lee YM, Choi H, Lee HM. Alterations of vasoactive intestinal polypeptide receptors in allergic rhinitis. Am J Rhinol Allergy. 2011; 25:e44–7.
Article33. Baraniuk JN, Castellino S, Lundgren JD, Goff J, Mullol J, Merida M, et al. Neuropeptide Y (NPY) in human nasal mucosa. Am J Respir Cell Mol Biol. 1990; 3:165–173.
Article34. Raap U, Braunstahl GJ. The role of neurotrophins in the pathophysiology of allergic rhinitis. Curr Opin Allergy Clin Immunol. 2010; 10:8–13.
Article35. Knipping S, Holzhausen HJ, Riederer A, Schrom T. Allergic and idiopathic rhinitis: an ultrastructural study. Eur Arch Otorhinolaryngol. 2009; 266:1249–1256.
Article36. Sanico AM, Stanisz AM, Gleeson TD, Bora S, Proud D, Bienenstock J, et al. Nerve growth factor expression and release in allergic inflammatory disease of the upper airways. Am J Respir Crit Care Med. 2000; 161:1631–1635.
Article37. Wang H, Song L, Ju W, Wang X, Dong L, Zhang Y, et al. The acute airway inflammation induced by PM2.5 exposure and the treatment of essential oils in Balb/c mice. Sci Rep. 2017; 7:44256.
Article38. De Falco G, Colarusso C, Terlizzi M, Popolo A, Pecoraro M, Commodo M, et al. Chronic obstructive pulmonary disease-derived circulating cells release IL-18 and IL-33 under ultrafine particulate matter exposure in a caspase-1/8-independent manner. Front Immunol. 2017; 8:1415.
Article39. Srivastava A, Sharma A, Yadav S, Flora SJ, Dwivedi UN, Parmar D. Gene expression profiling of candidate genes in peripheral blood mononuclear cells for predicting toxicity of diesel exhaust particles. Free Radic Biol Med. 2014; 67:188–194.
Article40. Chen B, Lu S, Li S, Wang B. Impact of fine particulate fluctuation and other variables on Beijing's air quality index. Environ Sci Pollut Res Int. 2015; 22:5139–5151.
Article41. Burte E, Leynaert B, Bono R, Brunekreef B, Bousquet J, Carsin AE, et al. Association between air pollution and rhinitis incidence in two European cohorts. Environ Int. 2018; 115:257–266.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MR study of normal nasal cycle
- The Effects of Alcohol on Nasal Patency and Mucociliary Clearance
- Change in the Nasal Patency and Mucociliary Clearance after Phenylephrine Spray
- Measurement of the so-called "Nasal Valve" in Japanese Subjects
- Intersession Repeatability of Acoustic Rhinometry Measurements in Healthy Volunteers