J Bacteriol Virol.
2019 Jun;49(2):69-80. 10.4167/jbv.2019.49.2.69.
Diverse Effects of Small Molecule Inhibitors on Actin Cytoskeleton Dynamics in HIV-1 Infection
- Affiliations
-
- 1Division of Viral Disease Research, Center for Infectious Disease Research, Korea National Institute of Health, Chungbuk, Republic of Korea. kmc755@korea.kr
- KMID: 2452048
- DOI: http://doi.org/10.4167/jbv.2019.49.2.69
Abstract
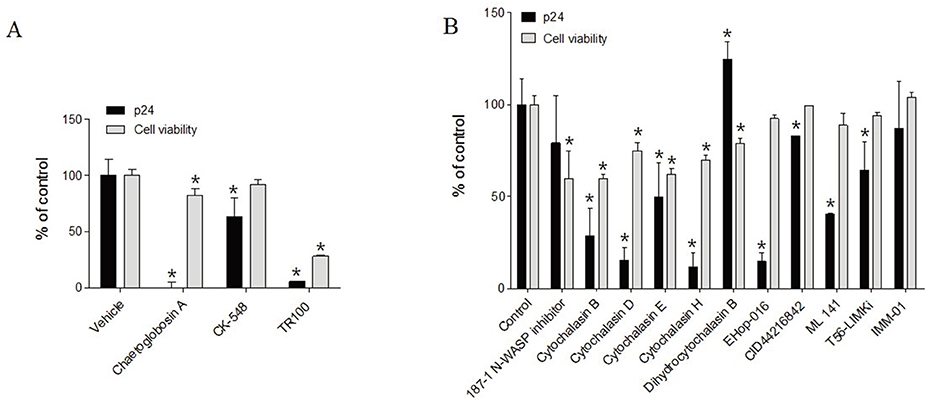
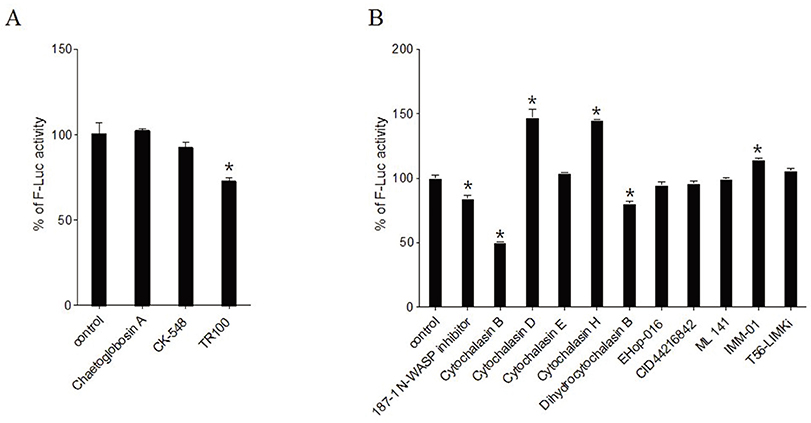
- The dynamics of the actin cytoskeleton plays a pivotal role in the process of cell division, the transportation of organelles, vesicle trafficking and cell movement. Human immunodeficiency virus type 1 (HIV-1) hijacks the actin dynamics network during the viral entry and migration of the pre-integration complex (PIC) into the nucleus. Actin dynamics linked to HIV-1 has emerged as a potent therapeutic target against HIV infection. Although some inhibitors have been intensely analyzed with regard to HIV-1 infection, their effects are sometimes disputed and the exact mechanisms for actin dynamics in HIV infection have not been well elucidated. In this study, the small molecules regulating HIV-1 infection from diverse inhibitors of the actin dynamic network were screened. Two compounds, including Chaetoglobosin A and CK-548, were observed to specifically bar the viral infection, while the cytochalasin family, 187-1, N-WASP inhibitor, Rho GTPase family inhibitors (EHop-016, CID44216842, and ML-141) and LIMK inhibitor (LIM domain kinase inhibitor) increased the viral infection without cytotoxicity within a range of ~ µM. However, previously known inhibitory compounds of HIV-1 infection, such as Latrunculin A, Jasplakinolide, Wiskostatin and Swinholide A, exhibited either an inhibitory effect on HIV-1 infection combined with severe cytotoxicity or showed no effects. Our data indicate that Chaetoglobosin A and CK-548 have considerable potential for development as new therapeutic drugs for the treatment of HIV infection. In addition, the newly identified roles of Cytochalasins and some inhibitors of Rho GTPase and LIMK may provide fundamental knowledge for understanding the complicated actin dynamic pathway when infected by HIV-1. Remarkably, the newly defined action modes of the inhibitors may be helpful in developing potent anti-HIV drugs that target the actin network, which are required for HIV infection.
MeSH Terms
Figure
Reference
-
1. Gallo RC, Salahuddin SZ, Popovic M, Shearer GM, Kaplan M, Haynes BF, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984; 224:500–503.
Article2. World Health Organization USCfDCaP, The Global Fund to Fight AIDS, Tuberculosis and Malaria. HIV drug resistance report 2017. World Health Organization;2017.3. Lee SH, Dominguez R. Regulation of actin cytoskeleton dynamics in cells. Mol Cells. 2010; 29:311–325.
Article4. Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev. 2014; 94:235–263.
Article5. Frischknecht F, Moreau V, Röttger S, Gonfloni S, Reckmann I, Superti-Furga G, et al. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999; 401:926–929.
Article6. Goley ED, Ohkawa T, Mancuso J, Woodruff JB, D'Alessio JA, Cande WZ, et al. Dynamic nuclear actin assembly by Arp2/3 complex and a baculovirus WASP-like protein. Science. 2006; 314:464–467.
Article7. Stolp B, Reichman-Fried M, Abraham L, Pan X, Giese SI, Hannemann S, et al. HIV-1 Nef interferes with host cell motility by deregulation of Cofilin. Cell Host Microbe. 2009; 6:174–186.
Article8. Spear M, Guo J, Wu Y. The trinity of the cortical actin in the initiation of HIV-1 infection. Retrovirology. 2012; 9:45.
Article9. Spear M, Guo J, Wu Y. Novel anti-HIV therapeutics targeting chemokine receptors and actin regulatory pathways. Immunol Rev. 2013; 256:300–312.
Article10. Yoder A, Yu D, Dong L, Iyer SR, Xu X, Kelly J, et al. HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell. 2008; 134:782–792.
Article11. Bukrinskaya A, Brichacek B, Mann A, Stevenson M. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J Exp Med. 1998; 188:2113–2125.
Article12. Carter GC, Bernstone L, Baskaran D, James W. HIV-1 infects macrophages by exploiting an endocytic route dependent on dynamin, Rac1 and Pak1. Virology. 2011; 409:234–250.
Article13. Haller C, Rauch S, Michel N, Hannemann S, Lehmann MJ, Keppler OT, et al. The HIV-1 pathogenicity factor Nef interferes with maturation of stimulatory T-lymphocyte contacts by modulation of N-Wasp activity. J Biol Chem. 2006; 281:19618–19630.
Article14. Kim HY, Choi BS, Kim SS, Roh TY, Park J, Yoon CH. NUCKS1, a novel Tat coactivator, plays a crucial role in HIV-1 replication by increasing Tat-mediated viral transcription on the HIV-1 LTR promoter. Retrovirology. 2014; 11:67.
Article15. Shin Y, Yoon CH, Yang HJ, Lim H, Choi BS, Kim SS, et al. Functional characteristics of the natural polymorphisms of HIV-1 gp41 in HIV-1 isolates from enfuvirtide-naive Korean patients. Arch Virol. 2016; 161:1547–1557.
Article16. Shin Y, Choi BS, Kim KC, Kang C, Kim K, Yoon CH. Development of a dual reporter screening assay for distinguishing the inhibition of HIV Tat-mediated transcription from off-target effects. J Virol Methods. 2017; 249:1–9.
Article17. Kawahara T, Itoh M, Izumikawa M, Sakata N, Tsuchida T, Shin-ya K. New chaetoglobosin derivatives, MBJ-0038, MBJ-0039 and MBJ-0040, isolated from the fungus Chaetomium sp. f24230o. J Antibiot (Tokyo). 2013; 66:727–730.
Article18. Nolen BJ, Tomasevic N, Russell A, Pierce DW, Jia Z, McCormick CD, et al. Characterization of two classes of small molecule inhibitors of Arp2/3 complex. Nature. 2009; 460:1031–1034.
Article19. Bonello TT, Janco M, Hook J, Byun A, Appaduray M, Dedova I, et al. A small molecule inhibitor of tropomyosin dissociates actin binding from tropomyosin-directed regulation of actin dynamics. Sci Rep. 2016; 6:19816.
Article20. Arden JD, Lavik KI, Rubinic KA, Chiaia N, Khuder SA, Howard MJ, et al. Small-molecule agonists of mammalian Diaphanous-related (mDia) formins reveal an effective glioblastoma anti-invasion strategy. Mol Biol Cell. 2015; 26:3704–3718.
Article21. De Clercq E, Li G. Approved Antiviral Drugs over the Past 50 Years. Clin Microbiol Rev. 2016; 29:695–747.
Article22. Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R. HIV reservoirs: what, where and how to target them. Nat Rev Microbiol. 2016; 14:55–60.
Article23. Margolis AM, Heverling H, Pham PA, Stolbach A. A review of the toxicity of HIV medications. J Med Toxicol. 2014; 10:26–39.
Article24. Vorster PJ, Guo J, Yoder A, Wang W, Zheng Y, Xu X, et al. LIM kinase 1 modulates cortical actin and CXCR4 cycling and is activated by HIV-1 to initiate viral infection. J Biol Chem. 2011; 286:12554–12564.
Article25. Spear M, Guo J, Turner A, Yu D, Wang W, Meltzer B, et al. HIV-1 triggers WAVE2 phosphorylation in primary CD4 T cells and macrophages, mediating Arp2/3-dependent nuclear migration. J Biol Chem. 2014; 289:6949–6959.
Article26. Sekita S, Yoshihira K, Natori S, Kuwano H. Structures of chaetoglobosin A and B, cytotoxic metabolites of chaetomium globosum. Tetrahedron Letters. 1973; 14:2109–2112.
Article27. Wang W, Guo J, Yu D, Vorster PJ, Chen W, Wu Y. A dichotomy in cortical actin and chemotactic actin activity between human memory and naive T cells contributes to their differential susceptibility to HIV-1 infection. J Biol Chem. 2012; 287:35455–35469.
Article28. Guerriero CJ, Weisz OA. N-WASP inhibitor wiskostatin nonselectively perturbs membrane transport by decreasing cellular ATP levels. Am J Physiol Cell Physiol. 2007; 292:C1562–C1566.
Article29. Jernigan KM, Blumenthal R, Puri A. Varying effects of temperature, Ca(2+) and cytochalasin on fusion activity mediated by human immunodeficiency virus type 1 and type 2 glycoproteins. FEBS Lett. 2000; 474:246–251.
Article30. Kondo N, Marin M, Kim JH, Desai TM, Melikyan GB. Distinct requirements for HIV-cell fusion and HIV-mediated cell-cell fusion. J Biol Chem. 2015; 290:6558–6573.
Article31. Frey S, Marsh M, Günther S, Pelchen-Matthews A, Stephens P, Ortlepp S, et al. Temperature dependence of cell-cell fusion induced by the envelope glycoprotein of human immunodeficiency virus type 1. J Virol. 1995; 69:1462–1472.
Article32. Iyengar S, Hildreth JE, Schwartz DH. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J Virol. 1998; 72:5251–5255.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Staurosporine and cytochalasin D induce chondrogenesis by regulation of actin dynamics in different way
- Molecular association of CD98, CD29, and CD147 critically mediates monocytic U937 cell adhesion
- Increased Fungal Infections while using Emerging Therapies (Biologics and Small-molecule Inhibitors) for Treating Skin Diseases: A Review
- Platelet Shape Changes and Cytoskeleton Dynamics as Novel Therapeutic Targets for Anti-Thrombotic Drugs
- Small GTPases and formins in mammalian oocyte maturation: cytoskeletal organizers