Blood Res.
2018 Jun;53(2):145-151. 10.5045/br.2018.53.2.145.
Allogeneic hematopoietic stem cell transplantation in congenital hemoglobinopathies with myeloablative conditioning and rabbit anti-thymocyte globulin
- Affiliations
-
- 1Department of Pediatrics, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. cngped@catholic.ac.kr
- 2Department of Pediatrics, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea.
- KMID: 2451033
- DOI: http://doi.org/10.5045/br.2018.53.2.145
Abstract
- BACKGROUND
Allogeneic hematopoietic stem cell transplantation (HSCT) is a curative therapy for β-thalassemia major (TM) and sickle cell disease (SCD) in children. Graft-versus-host disease (GVHD) and treatment-related mortality (TRM) remain significant challenges to improving survival after HSCT. Here, we analyzed the outcome of TM and SCD patients, who received allogeneic HSCT with myeloablative conditioning at our institution.
METHODS
Twenty-two patients (15 TM, 7 SCD), with a median age of 9 years (range, 1.6-16.9), underwent allogeneic HSCT using busulfan, cyclophosphamide and rabbit anti-thymocyte globulin-based conditioning. Cells were derived from either the bone marrow (8 patients), or peripheral blood stem cells (14 patients). The majority of patients received HSCT from a matched sibling donor (N=18). GVHD prophylaxis included cyclosporine and short course methotrexate.
RESULTS
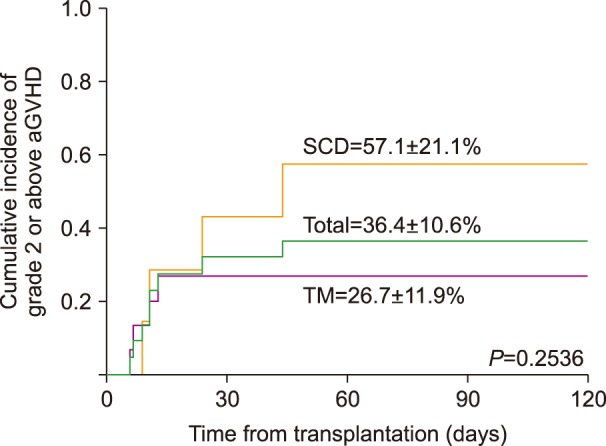
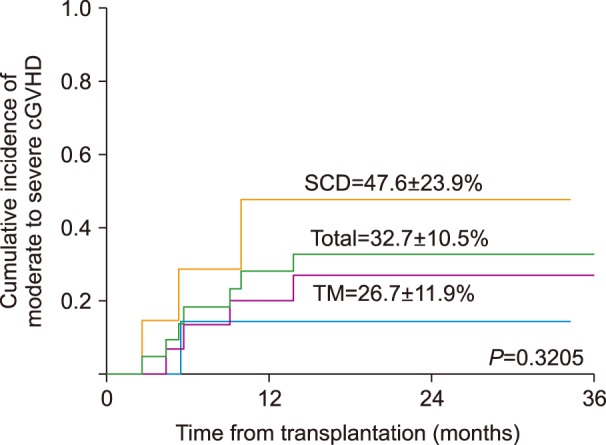
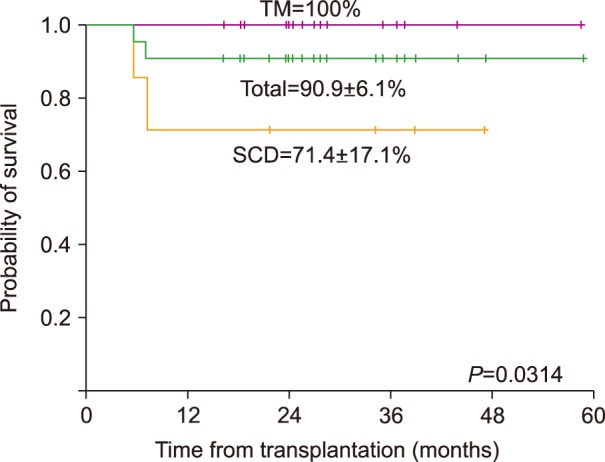
All patients achieved donor engraftment. Two SCD patients died from TRM-related grade IV gut GVHD (N=1) or severe bronchiolitis obliterans (BO) (N=1). Cumulative incidence of acute and chronic GVHD was 36.4% and 32.7%, respectively. Veno-occlusive disease (VOD) occurred in 8 patients (36.4%), but resolved in all instances. Epstein-Barr virus (EBV)-related post-transplantation lymphoproliferative disease (PTLD) occurred in 1 patient. The overall survival (OS) was 90.9% (TM 100%, SCD 71.4%), with all patients achieving transfusion independence, while 8 achieved complete donor chimerism.
CONCLUSION
Busulfan, cyclophosphamide, and ATG-based conditioning for HSCT of TM and SCD patients did not result in graft failure, although modifications may be required to reduce VOD incidence. Further changes to donor type and cell source prioritization are necessary to minimize TRM and morbidity caused by GVHD.
Keyword
MeSH Terms
-
Anemia, Sickle Cell
Antilymphocyte Serum*
beta-Thalassemia
Bone Marrow
Bronchiolitis Obliterans
Busulfan
Child
Chimerism
Cyclophosphamide
Cyclosporine
Graft vs Host Disease
Hematopoietic Stem Cell Transplantation*
Hematopoietic Stem Cells*
Hemoglobinopathies*
Herpesvirus 4, Human
Humans
Incidence
Methotrexate
Mortality
Siblings
Stem Cells
Tissue Donors
Transplants
Antilymphocyte Serum
Busulfan
Cyclophosphamide
Cyclosporine
Methotrexate
Figure
Cited by 1 articles
-
Is Treosulfan-Based Conditioning Attractive as a Reduced-Intensity Conditioning Regimen in Korea?
Se Hyung Kim, Young Sok Ji, Jina Yun, Seong Hyeok Choi, Sung Hee Lim, Chan Kyu Kim, Seong Kyu Park
J Korean Med Sci. 2023;38(36):e281. doi: 10.3346/jkms.2023.38.e281.
Reference
-
1. Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008; 86:480–487. PMID: 18568278.
Article2. Weatherall DJ, Williams TN, Allen SJ, O'Donnell A. The population genetics and dynamics of the thalassemias. Hematol Oncol Clin North Am. 2010; 24:1021–1031. PMID: 21075278.
Article3. Park ES, Jung HL, Kim HJ, et al. Hereditary hemolytic anemia in Korea from 2007 to 2011: A study by the Korean Hereditary Hemolytic Anemia Working Party of the Korean Society of Hematology. Blood Res. 2013; 48:211–216. PMID: 24086942.
Article4. Cavazzana-Calvo M, Payen E, Negre O, et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010; 467:318–322. PMID: 20844535.
Article5. Srivastava A, Shaji RV. Cure for thalassemia major - from allogeneic hematopoietic stem cell transplantation to gene therapy. Haematologica. 2017; 102:214–223. PMID: 27909215.
Article6. Malik P. Gene therapy for hemoglobinopathies: tremendous successes and remaining caveats. Mol Ther. 2016; 24:668–670. PMID: 27081721.
Article7. Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995; 15:825–828. PMID: 7581076.8. Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015; 21:389–401.e1. PMID: 25529383.9. Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007; 40:381–387. PMID: 17563735.
Article10. Rodgers GP, Dover GJ, Noguchi CT, Schechter AN, Nienhuis AW. Hematologic responses of patients with sickle cell disease to treatment with hydroxyurea. N Engl J Med. 1990; 322:1037–1045. PMID: 1690857.
Article11. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014; 124:3850–3857. PMID: 25287707.
Article12. Talano JA, Cairo MS. Hematopoietic stem cell transplantation for sickle cell disease: state of the science. Eur J Haematol. 2015; 94:391–399. PMID: 25200500.
Article13. Iannone R, Casella JF, Fuchs EJ, et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta-thalassemia. Biol Blood Marrow Transplant. 2003; 9:519–528. PMID: 12931121.14. Goussetis E, Peristeri I, Kitra V, et al. HLA-matched sibling stem cell transplantation in children with β-thalassemia with anti-thymocyte globulin as part of the preparative regimen: the Greek experience. Bone Marrow Transplant. 2012; 47:1061–1066. PMID: 22080966.
Article15. Bernaudin F, Socie G, Kuentz M, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007; 110:2749–2756. PMID: 17606762.
Article16. Soni S, Gross TG, Rangarajan H, Baker KS, Sturm M, Rhodes M. Outcomes of matched sibling donor hematopoietic stem cell transplantation for severe sickle cell disease with myeloablative conditioning and intermediate-dose of rabbit anti-thymocyte globulin. Pediatr Blood Cancer. 2014; 61:1685–1689. PMID: 24740582.
Article17. Cheuk DK, Wang P, Lee TL, et al. Risk factors and mortality predictors of hepatic veno-occlusive disease after pediatric hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007; 40:935–944. PMID: 17768390.
Article18. Jastaniah W, Harmatz P, Pakbaz Z, Fischer R, Vichinsky E, Walters MC. Transfusional iron burden and liver toxicity after bone marrow transplantation for acute myelogenous leukemia and hemoglobinopathies. Pediatr Blood Cancer. 2008; 50:319–324. PMID: 17557314.
Article19. Coppell JA, Richardson PG, Soiffer R, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010; 16:157–168. PMID: 19766729.
Article20. Brachet C, Heinrichs C, Tenoutasse S, Devalck C, Azzi N, Ferster A. Children with sickle cell disease: growth and gonadal function after hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2007; 29:445–450. PMID: 17609621.
Article21. Shenoy S, Angelucci E, Arnold SD, et al. Current results and future research priorities in late effects after hematopoietic stem cell transplantation for children with sickle cell disease and thalassemia: a consensus statement from the second pediatric blood and marrow transplant consortium international conference on late effects after pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2017; 23:552–561. PMID: 28065838.
Article22. Locatelli F, Kabbara N, Ruggeri A, et al. Outcome of patients with hemoglobinopathies given either cord blood or bone marrow transplantation from an HLA-identical sibling. Blood. 2013; 122:1072–1078. PMID: 23692854.
Article23. Bernardo ME, Piras E, Vacca A, et al. Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood. 2012; 120:473–476. PMID: 22645178.
Article24. Lucarelli G, Gaziev J. Advances in the allogeneic transplantation for thalassemia. Blood Rev. 2008; 22:53–63. PMID: 18039551.
Article25. Ghavamzadeh A, Iravani M, Ashouri A, et al. Peripheral blood versus bone marrow as a source of hematopoietic stem cells for allogeneic transplantation in children with class I and II beta thalassemia major. Biol Blood Marrow Transplant. 2008; 14:301–308. PMID: 18275896.
Article26. Ruggeri A, Eapen M, Scaravadou A, et al. Umbilical cord blood transplantation for children with thalassemia and sickle cell disease. Biol Blood Marrow Transplant. 2011; 17:1375–1382. PMID: 21277376.
Article27. Bolaños-Meade J, Fuchs EJ, Luznik L, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012; 120:4285–4291. PMID: 22955919.
Article28. Sodani P, Isgrò A, Gaziev J, et al. Purified T-depleted, CD34+ peripheral blood and bone marrow cell transplantation from haploidentical mother to child with thalassemia. Blood. 2010; 115:1296–1302. PMID: 19897573.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Successful Allogeneic Hematopoietic Stem Cell Transplantation for a Patient with Very Severe Aplastic Anemia During Active Invasive Fungal Infection
- Allogeneic Stem Cell Transplantation for Patients with Advanced Hematological Malignancies: Comparison of Fludarabine-based Reduced Intensity Conditioning versus Myeloablative Conditioning
- Graft-versus-Leukemia Effect of Nonmyeloablative Stem Cell Transplantation
- Allogeneic hematopoietic stem cell transplantation for myelodysplastic syndromes
- Treosulfan-Based Conditioning Regimen for Hematopoietic Stem Cell Transplantation in Pediatric Patients with Hemophagocytic Lymphohistiocytosis