J Breast Cancer.
2019 Jun;22(2):155-171. 10.4048/jbc.2019.22.e26.
Insights into Hypoxia: Non-invasive Assessment through Imaging Modalities and Its Application in Breast Cancer
- Affiliations
-
- 1Department of Radiology, Breast Imaging Service, Memorial Sloan Kettering Cancer Center, New York, NY, USA. idaimiel@hotmail.com
- KMID: 2450112
- DOI: http://doi.org/10.4048/jbc.2019.22.e26
Abstract
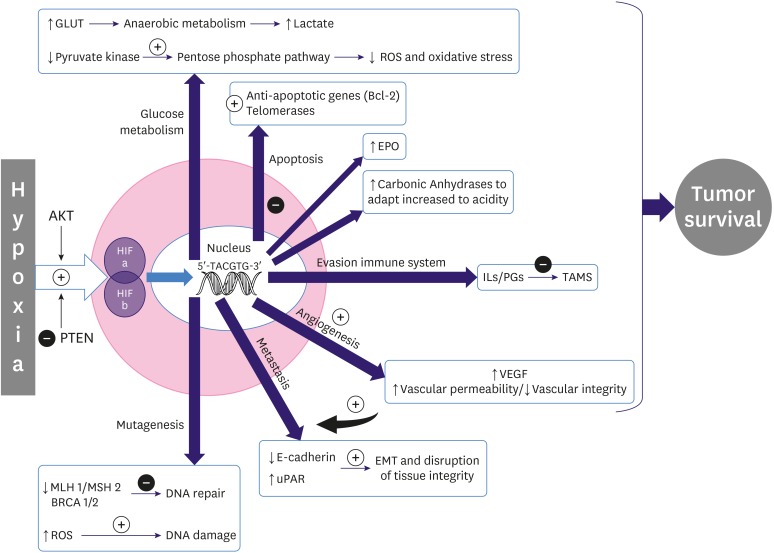
- Oxygen is crucial to maintain the homeostasis in aerobic cells. Hypoxia is a condition in which cells are deprived of the oxygen supply necessary for their optimum performance. Whereas oxygen deprivation may occur in normal physiological processes, hypoxia is frequently associated with pathological conditions. It has been identified as a stressor in the tumor microenvironment, acting as a key mediator of cancer development. Numerous pathways are activated in hypoxic cells that affect cell signaling and gene regulation to promote the survival of these cells by stimulating angiogenesis, switching cellular metabolism, slowing their growth rate, and preventing apoptosis. The induction of dysregulated metabolism in cancer cells by hypoxia results in aggressive tumor phenotypes that are characterized by rapid progression, treatment resistance, and poor prognosis. A non-invasive assessment of hypoxia-induced metabolic and architectural changes in tumors is advisable to fully improve breast cancer (BC) patient management, by potentially reducing the need for invasive biopsy procedures and evaluating tumor response to treatment. This review provides a comprehensive overview of the molecular changes in breast tumors secondary to hypoxia and the non-invasive imaging alternatives to evaluate oxygen deprivation, with an emphasis on their application in BC and the advantages and limitations of the currently available techniques.
Keyword
MeSH Terms
Figure
Reference
-
1. Hultén LM, Levin M. The role of hypoxia in atherosclerosis. Curr Opin Lipidol. 2009; 20:409–414. PMID: 19644366.
Article2. Taylor PC, Sivakumar B. Hypoxia and angiogenesis in rheumatoid arthritis. Curr Opin Rheumatol. 2005; 17:293–298. PMID: 15838239.
Article3. Ishida S, Shinoda K, Kawashima S, Oguchi Y, Okada Y, Ikeda E. Coexpression of VEGF receptors VEGF-R2 and neuropilin-1 in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000; 41:1649–1656. PMID: 10845581.4. Mateo J, Izquierdo-Garcia D, Badimon JJ, Fayad ZA, Fuster V. Noninvasive assessment of hypoxia in rabbit advanced atherosclerosis using 18f-fluoromisonidazole PET imaging. Circ Cardiovasc Imaging. 2014; 7:312–320. PMID: 24508668.5. Fu Q, Colgan SP, Shelley CS. Hypoxia: the force that drives chronic kidney disease. Clin Med Res. 2016; 14:15–39. PMID: 26847481.
Article6. Bonnitcha P, Grieve S, Figtree G. Clinical imaging of hypoxia: current status and future directions. Free Radic Biol Med. 2018; 126:296–312. PMID: 30130569.
Article7. Vaupel P, Mayer A, Höckel M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004; 381:335–354. PMID: 15063685.
Article8. Wallace TE, Patterson AJ, Abeyakoon O, Bedair R, Manavaki R, McLean MA, et al. Detecting gas-induced vasomotor changes via blood oxygenation level-dependent contrast in healthy breast parenchyma and breast carcinoma. J Magn Reson Imaging. 2016; 44:335–345. PMID: 26898173.
Article9. Vaupel P. Prognostic potential of the pre-therapeutic tumor oxygenation status. Adv Exp Med Biol. 2009; 645:241–246. PMID: 19227477.
Article10. Gilkes DM. Implications of hypoxia in breast cancer metastasis to bone. Int J Mol Sci. 2016; 17:E1669. PMID: 27706047.
Article11. Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006; 7:359–371. PMID: 16633338.
Article12. Lu H, Shu XO, Cui Y, Kataoka N, Wen W, Cai Q, et al. Association of genetic polymorphisms in the VEGF gene with breast cancer survival. Cancer Res. 2005; 65:5015–5019. PMID: 15958542.
Article13. Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002; 29:15–18.
Article14. Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002; 2:38–47. PMID: 11902584.15. Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004; 9(Suppl 5):4–9.
Article16. Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008; 8:180–192. PMID: 18273037.17. Shaw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006; 18:598–608. PMID: 17046224.
Article18. Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999; 24:68–72. PMID: 10098401.
Article19. Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda). 2004; 19:176–182. PMID: 15304631.
Article20. Adamaki M, Georgountzou A, Moschovi M. Cancer and the cellular response to hypoxia. Pediatr Therapeut. 2012; S1:002.
Article21. Gatenby RA, Smallbone K, Maini PK, Rose F, Averill J, Nagle RB, et al. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br J Cancer. 2007; 97:646–653. PMID: 17687336.
Article22. Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, et al. Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in Human breast cancer. Cancer Res. 2002; 62:1326–1329. PMID: 11888900.23. Carnero A, Lleonart M. The hypoxic microenvironment: a determinant of cancer stem cell evolution. BioEssays. 2016; 38(Suppl 1):S65–S74. PMID: 27417124.
Article24. Harrison L, Blackwell K. Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist. 2004; 9(Suppl 5):31–40. PMID: 15591420.
Article25. Terry S, Faouzi Zaarour R, Hassan Venkatesh G, Francis A, El-Sayed W, Buart S, et al. Role of hypoxic stress in regulating tumor immunogenicity, resistance and plasticity. Int J Mol Sci. 2018; 19:E3044. PMID: 30301213.
Article26. Nardinocchi L, Puca R, Sacchi A, D'Orazi G. Inhibition of HIF-1alpha activity by homeodomain-interacting protein kinase-2 correlates with sensitization of chemoresistant cells to undergo apoptosis. Mol Cancer. 2009; 8:1. PMID: 19128456.
Article27. De Francesco EM, Maggiolini M, Tanowitz HB, Sotgia F, Lisanti MP. Targeting hypoxic cancer stem cells (CSCs) with Doxycycline: implications for optimizing anti-angiogenic therapy. Oncotarget. 2017; 8:56126–56142. PMID: 28915578.
Article28. Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953; 26:638–648. PMID: 13106296.
Article29. Colliez F, Gallez B, Jordan BF. Assessing tumor oxygenation for predicting outcome in radiation oncology: a review of studies correlating tumor hypoxic status and outcome in the preclinical and clinical settings. Front Oncol. 2017; 7:10. PMID: 28180110.
Article30. Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007; 25:4066–4074. PMID: 17827455.
Article31. Vordermark D, Horsman MR. Hypoxia as a biomarker and for personalized radiation oncology. Recent Results Cancer Res. 2016; 198:123–142. PMID: 27318684.
Article32. Wardman P. Chemical radiosensitizers for use in radiotherapy. Clin Oncol (R Coll Radiol). 2007; 19:397–417. PMID: 17478086.
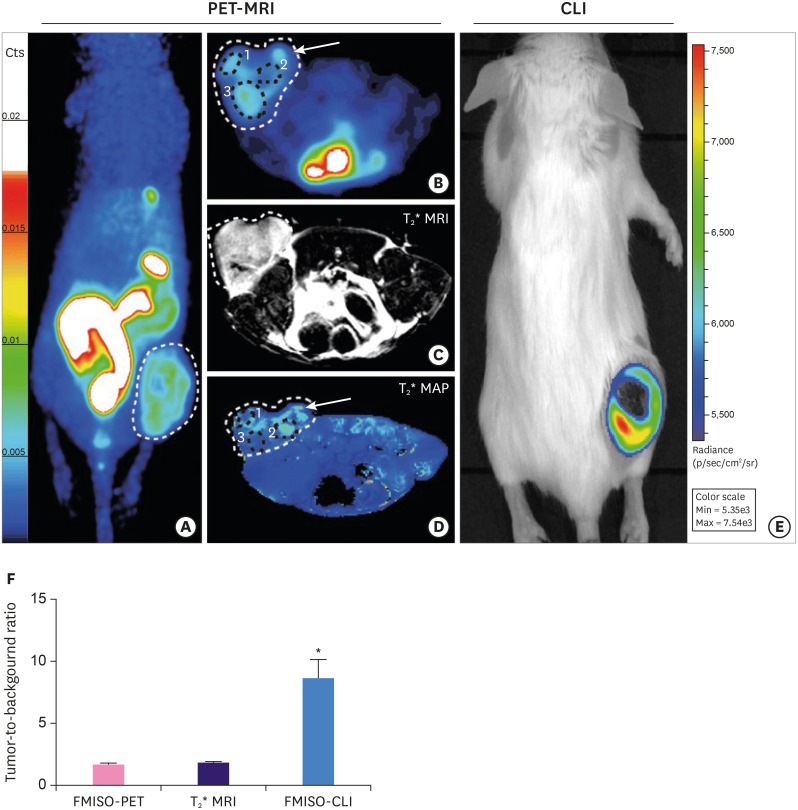
Article33. Fleming IN, Manavaki R, Blower PJ, West C, Williams KJ, Harris AL, et al. Imaging tumour hypoxia with positron emission tomography. Br J Cancer. 2015; 112:238–250. PMID: 25514380.
Article34. Ueda S, Saeki T, Osaki A, Yamane T, Kuji I. Bevacizumab induces acute hypoxia and cancer progression in patients with refractory breast cancer: multimodal functional imaging and multiplex cytokine analysis. Clin Cancer Res. 2017; 23:5769–5778. PMID: 28679773.
Article35. Quintela-Fandino M, Lluch A, Manso L, Calvo I, Cortes J, García-Saenz JA, et al. 18F-fluoromisonidazole PET and activity of neoadjuvant nintedanib in early HER2-negative breast cancer: a window-of-opportunity randomized trial. Clin Cancer Res. 2017; 23:1432–1441. PMID: 27587436.36. Cheng J, Lei L, Xu J, Sun Y, Zhang Y, Wang X, et al. 18F-fluoromisonidazole PET/CT: a potential tool for predicting primary endocrine therapy resistance in breast cancer. J Nucl Med. 2013; 54:333–340. PMID: 23401605.37. Hypoxia-positron emission tomography (PET) and intensity modulated proton therapy (IMPT) dose painting in patients with chordomas. ClinicalTrials.gov Identifier: NCT00713037. 2017. U.S. National Library of Medicine;https://clinicaltrials.gov/ct2/show/NCT00713037.38. Reischl G, Dorow DS, Cullinane C, Katsifis A, Roselt P, Binns D, et al. Imaging of tumor hypoxia with [124I]IAZA in comparison with [18F]FMISO and [18F]FAZA--first small animal PET results. J Pharm Pharm Sci. 2007; 10:203–211. PMID: 17706178.39. Grönroos T, Bentzen L, Marjamäki P, Murata R, Horsman MR, Keiding S, et al. Comparison of the biodistribution of two hypoxia markers [18F]FETNIM and [18F]FMISO in an experimental mammary carcinoma. Eur J Nucl Med Mol Imaging. 2004; 31:513–520. PMID: 14722675.40. Xu Z, Li XF, Zou H, Sun X, Shen B. 18F-Fluoromisonidazole in tumor hypoxia imaging. Oncotarget. 2017; 8:94969–94979. PMID: 29212283.41. Vāvere AL, Lewis JS. Cu-ATSM: a radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans. 2007; (43):4893–4902. PMID: 17992274.
Article42. Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012; 9:674–687. PMID: 23149893.
Article43. Challapalli A, Carroll L, Aboagye EO. Molecular mechanisms of hypoxia in cancer. Clin Transl Imaging. 2017; 5:225–253. PMID: 28596947.
Article44. Pinker K, Helbich TH, Morris EA. The potential of multiparametric MRI of the breast. Br J Radiol. 2017; 90:20160715. PMID: 27805423.
Article45. Jensen RL, Mumert ML, Gillespie DL, Kinney AY, Schabel MC, Salzman KL. Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro-oncol. 2014; 16:280–291. PMID: 24305704.
Article46. Halle C, Andersen E, Lando M, Aarnes EK, Hasvold G, Holden M, et al. Hypoxia-induced gene expression in chemoradioresistant cervical cancer revealed by dynamic contrast-enhanced MRI. Cancer Res. 2012; 72:5285–5295. PMID: 22890239.
Article47. Baudelet C, Gallez B. How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn Reson Med. 2002; 48:980–986. PMID: 12465107.48. Christen T, Lemasson B, Pannetier N, Farion R, Remy C, Zaharchuk G, et al. Is T2* enough to assess oxygenation? Quantitative blood oxygen level-dependent analysis in brain tumor. Radiology. 2012; 262:495–502. PMID: 22156990.
Article49. Howe FA, Robinson SP, Rodrigues LM, Griffiths JR. Flow and oxygenation dependent (FLOOD) contrast MR imaging to monitor the response of rat tumors to carbogen breathing. Magn Reson Imaging. 1999; 17:1307–1318. PMID: 10576716.50. Mason RP, Zhao D, Pacheco-Torres J, Cui W, Kodibagkar VD, Gulaka PK, et al. Multimodality imaging of hypoxia in preclinical settings. Q J Nucl Med Mol Imaging. 2010; 54:259–280. PMID: 20639813.51. White DA, Zhang Z, Li L, Gerberich J, Stojadinovic S, Peschke P, et al. Developing oxygen-enhanced magnetic resonance imaging as a prognostic biomarker of radiation response. Cancer Lett. 2016; 380:69–77. PMID: 27267808.
Article52. Ding Y, Mason RP, McColl RW, Yuan Q, Hallac RR, Sims RD, et al. Simultaneous measurement of tissue oxygen level-dependent (TOLD) and blood oxygenation level-dependent (BOLD) effects in abdominal tissue oxygenation level studies. J Magn Reson Imaging. 2013; 38:1230–1236. PMID: 23749420.
Article53. O'Connor JP, Robinson SP, Waterton JC. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. Br J Radiol. 2019; 92:20180642. PMID: 30272998.54. Maril N, Collins CM, Greenman RL, Lenkinski RE. Strategies for shimming the breast. Magn Reson Med. 2005; 54:1139–1145. PMID: 16217775.
Article55. Rakow-Penner R, Daniel B, Glover GH. Detecting blood oxygen level-dependent (BOLD) contrast in the breast. J Magn Reson Imaging. 2010; 32:120–129. PMID: 20578018.
Article56. Liu M, Guo X, Wang S, Jin M, Wang Y, Li J, et al. BOLD-MRI of breast invasive ductal carcinoma: correlation of R2* value and the expression of HIF-1α. Eur Radiol. 2013; 23:3221–3227. PMID: 23835924.
Article57. Choi HY, Ko ES, Han BK, Kim EJ, Kim SM, Lim Y, et al. Prognostic significance of transverse relaxation rate (R2*) in blood oxygenation level-dependent magnetic resonance imaging in patients with invasive breast cancer. PLoS One. 2016; 11:e0158500. PMID: 27384310.
Article58. Hunjan S, Zhao D, Constantinescu A, Hahn EW, Antich PP, Mason RP. Tumor oximetry: demonstration of an enhanced dynamic mapping procedure using fluorine-19 echo planar magnetic resonance imaging in the Dunning prostate R3327-AT1 rat tumor. Int J Radiat Oncol Biol Phys. 2001; 49:1097–1108. PMID: 11240252.
Article59. Zhao D, Jiang L, Hahn EW, Mason RP. Comparison of 1H blood oxygen level-dependent (BOLD) and 19F MRI to investigate tumor oxygenation. Magn Reson Med. 2009; 62:357–364. PMID: 19526495.60. Le D, Mason RP, Hunjan S, Constantinescu A, Barker BR, Antich PP. Regional tumor oxygen dynamics: 19F PBSR EPI of hexafluorobenzene. Magn Reson Imaging. 1997; 15:971–981. PMID: 9322216.
Article61. Procissi D, Claus F, Burgman P, Koziorowski J, Chapman JD, Thakur SB, et al. In vivo 19F magnetic resonance spectroscopy and chemical shift imaging of tri-fluoro-nitroimidazole as a potential hypoxia reporter in solid tumors. Clin Cancer Res. 2007; 13:3738–3747. PMID: 17575240.62. Zhang C, Moonshi SS, Wang W, Ta HT, Han Y, Han FY, et al. High F-content perfluoropolyether-based nanoparticles for targeted detection of breast cancer by 19F magnetic resonance and optical imaging. ACS Nano. 2018; 12:9162–9176. PMID: 30118590.63. Makela AV, Foster PJ. Imaging macrophage distribution and density in mammary tumors and lung metastases using fluorine-19 MRI cell tracking. Magn Reson Med. 2018; 80:1138–1147. PMID: 29327789.
Article64. Bartusik D, Tomanek B, Siluk D, Kaliszan R, Fallone G. The application of 19F magnetic resonance ex vivo imaging of three-dimensional cultured breast cancer cells to study the effect of δ-tocopherol. Anal Biochem. 2009; 387:315–317. PMID: 19454246.65. O'Flynn EA, DeSouza NM. Functional magnetic resonance: biomarkers of response in breast cancer. Breast Cancer Res. 2011; 13:204. PMID: 21392409.66. Velan SS, Spencer RG, Zweier JL, Kuppusamy P. Electron paramagnetic resonance oxygen mapping (EPROM): direct visualization of oxygen concentration in tissue. Magn Reson Med. 2000; 43:804–809. PMID: 10861874.
Article67. Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. J Nucl Med. 2008; 49(Suppl 2):129S–148S. PMID: 18523070.
Article68. Krishna MC, Matsumoto S, Yasui H, Saito K, Devasahayam N, Subramanian S, et al. Electron paramagnetic resonance imaging of tumor pO2. Radiat Res. 2012; 177:376–386. PMID: 22332927.69. Roussakis E, Li Z, Nichols AJ, Evans CL. Oxygen-sensing methods in biomedicine from the macroscale to the microscale. Angew Chem Int Ed Engl. 2015; 54:8340–8362. PMID: 26084034.
Article70. Shibata T, Giaccia AJ, Brown JM. Development of a hypoxia-responsive vector for tumor-specific gene therapy. Gene Ther. 2000; 7:493–498. PMID: 10757022.
Article71. Vordermark D, Shibata T, Brown JM. Green fluorescent protein is a suitable reporter of tumor hypoxia despite an oxygen requirement for chromophore formation. Neoplasia. 2001; 3:527–534. PMID: 11774035.
Article72. Harada H, Kizaka-Kondoh S, Hiraoka M. Optical imaging of tumor hypoxia and evaluation of efficacy of a hypoxia-targeting drug in living animals. Mol Imaging. 2005; 4:182–193. PMID: 16194450.
Article73. Blackmore KM, Knight JA, Walter J, Lilge L. The association between breast tissue optical content and mammographic density in pre- and post-menopausal women. PLoS One. 2015; 10:e0115851. PMID: 25590139.
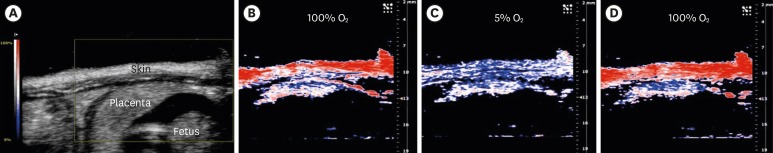
Article74. Wang LV, Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012; 335:1458–1462. PMID: 22442475.75. Lungu GF, Li ML, Xie X, Wang LV, Stoica G. In vivo imaging and characterization of hypoxia-induced neovascularization and tumor invasion. Int J Oncol. 2007; 30:45–54. PMID: 17143511.76. Arthuis CJ, Novell A, Raes F, Escoffre JM, Lerondel S, Le Pape A, et al. Real-time monitoring of placental oxygenation during maternal hypoxia and hyperoxygenation using photoacoustic imaging. PLoS One. 2017; 12:e0169850. PMID: 28081216.
Article77. Nam HS, Yoo H. Spectroscopic optical coherence tomography: a review of concepts and biomedical applications. Appl Spectrosc Rev. 2018; 53:91–111.
Article78. Reddy N, Nguyen BT. The utility of optical coherence tomography for diagnosis of basal cell carcinoma: a quantitative review. Br J Dermatol. 2019; 180:475–483. PMID: 30216419.
Article79. Neupane R, Gaudana R, Boddu SH. Imaging techniques in the diagnosis and management of ocular tumors: prospects and challenges. AAPS J. 2018; 20:97. PMID: 30187172.
Article80. Ha R, Friedlander LC, Hibshoosh H, Hendon C, Feldman S, Ahn S, et al. Optical coherence tomography: a novel imaging method for post-lumpectomy breast margin assessment-a multi-reader study. Acad Radiol. 2018; 25:279–287. PMID: 29174226.81. Singla N, Dubey K, Srivastava V. Automated assessment of breast cancer margin in optical coherence tomography images via pretrained convolutional neural network. J Biophotonics. 2019; 12:e201800255. PMID: 30318761.
Article82. Schuman JS. Spectral domain optical coherence tomography for glaucoma (an AOS thesis). Trans Am Ophthalmol Soc. 2008; 106:426–458. PMID: 19277249.83. Sandhu S, Kydd L, Jaworski J. Luminescent probe based techniques for hypoxia imaging. J Nanomed Res. 2017; 6:00160. PMID: 30417104.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic Utility of Artificial Intelligence in Breast Ultrasound
- Optical Imaging of the Breast
- Recent Progress in Synthesis of99m Tc‑labeled Complexes with Nitroimidazoles as SPECT Probes for Targeting Tumor Hypoxia
- A Rare Case of Male Primary Breast Lymphoma
- Nodular Metastatic Carcinoma from Invasive Lobular Breast Cancer