Int J Stem Cells.
2019 Mar;12(1):73-83. 10.15283/ijsc18094.
Predictive Role of Circulating Immune Cell Subtypes Early after Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Acute Leukemia
- Affiliations
-
- 1Department of Hematology, Seoul St. Mary's Hematology Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. ckmin@catholic.ac.kr
- 2Leukemia Research Institute, The Catholic University of Korea, Seoul, Korea.
- 3Department of Hematology, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea.
- 4Department of Hematology, Yeoido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2447226
- DOI: http://doi.org/10.15283/ijsc18094
Abstract
- BACKGROUND AND OBJECTIVES
Cells of innate immunity normally recover in the first weeks to months after allogenenic hematopoietic stem cell transplantation (allo-HSCT). Their relevance in terms of graft-versus-host disease (GVHD) and graft-versus-leukemia (GVL) effect is largely unknown. The predictive role of early recovery in the immune cells on acute GVHD and GVL effect after allo-HSCT was investigated in patients with acute leukemia who achieved the first complete remission.
METHODS
Peripheral blood samples were taken at the median of 14 days (range, 12~29 days) after allo-HSCT. A cohort including 119 samples and characteristics of patients were analyzed. Immune cell populations were identified by flow cytometry.
RESULTS
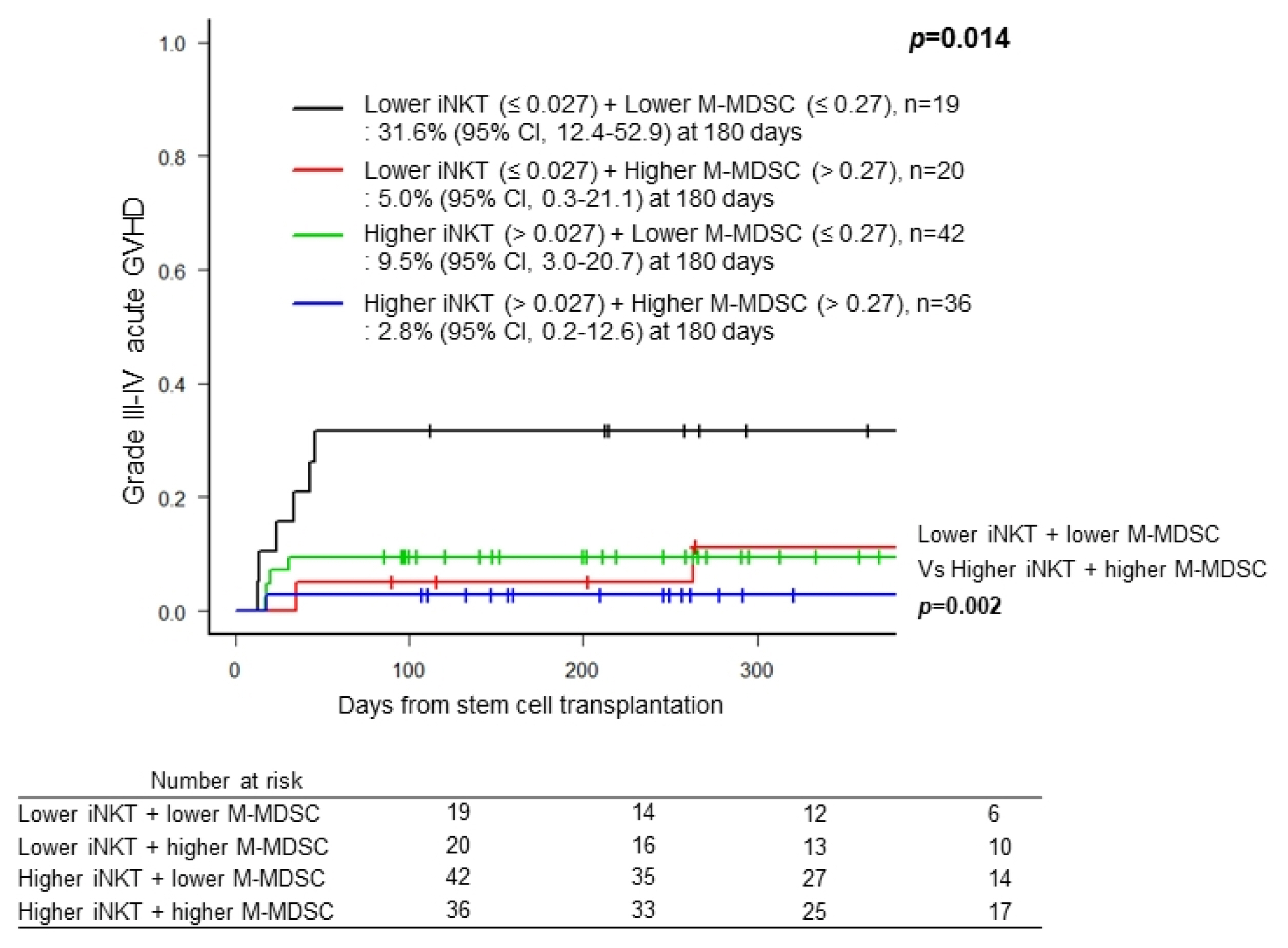
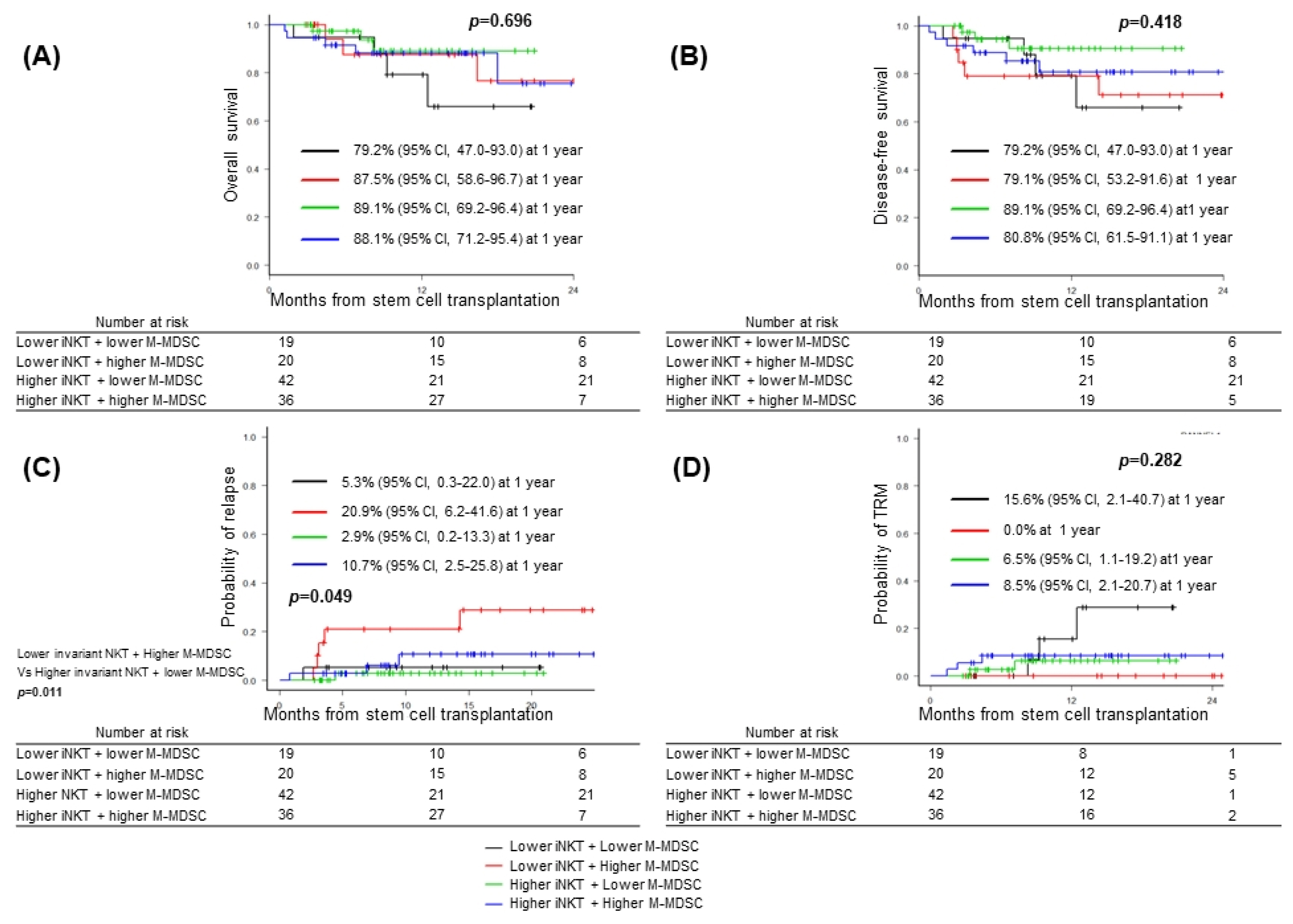
The median age was 49.0 years (range, 21~69) at transplantation. Univariate analysis showed that age less than 40 years old, lower frequencies of CD8+ T cells, invariant natural killer T (iNKT) cells, monocytic myeloid derived suppressor cells (M-MDSCs) and higher frequency of immature MDSCs were associated with occurrence of grade III-IV acute GVHD. Multivariate analyses showed that iNKT cells (hazard ratio (HR), 0.453, 95% CI, 0.091~0.844, p=0.024) and M-MDSCs (HR, 0.271, 95% CI, 0.078~0.937, p=0.039) were independent factors. Combination of higher frequencies of both cell subsets was associated with lower incidence of grade III-IV acute GVHD, whereas patients with lower frequency of iNKT cells and higher frequency of M-MDSCs showed significant higher probability of relapse.
CONCLUSIONS
iNKT cells and M-MDSCs could be relevant cell biomarkers for predicting acute GVHD and/or relapse in acute leukemia patients treated with allo-HSCT.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Janikova A, Bortlicek Z, Campr V, Kopalova N, Benesova K, Hamouzova M, Belada D, Prochazka V, Pytlik R, Vokurka S, Pirnos J, Duras J, Mocikova H, Mayer J, Trneny M. The incidence of biopsy-proven transformation in follicular lymphoma in the rituximab era. A retrospective analysis from the Czech Lymphoma Study Group (CLSG) database. Ann Hematol. 2018; 97:669–678. DOI: 10.1007/s00277-017-3218-0. PMID: 29318369.
Article2. Stelljes M, Krug U, Beelen DW, Braess J, Sauerland MC, Heinecke A, Ligges S, Sauer T, Tschanter P, Thoennissen GB, Berning B, Kolb HJ, Reichle A, Holler E, Schwerdtfeger R, Arnold R, Scheid C, Müller-Tidow C, Woermann BJ, Hiddemann W, Berdel WE, Büchner T. Allogeneic transplantation versus chemotherapy as post-remission therapy for acute myeloid leukemia: a prospective matched pairs analysis. J Clin Oncol. 2014; 32:288–296. DOI: 10.1200/JCO.2013.50.5768. PMID: 24366930.
Article3. Kim N, Cho SG. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells. 2015; 8:54–68. DOI: 10.15283/ijsc.2015.8.1.54. PMID: 26019755. PMCID: 4445710.
Article4. Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, Wadleigh M, DeAngelo DJ, Stone RM, Sakamaki H, Appelbaum FR, Döhner H, Antin JH, Soiffer RJ, Cutler C. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009; 301:2349–2361. DOI: 10.1001/jama.2009.813. PMID: 19509382. PMCID: 3163846.
Article5. Bourlon C, Lacayo-Leñero D, Inclán-Alarcón SI, Demichelis-Gómez R. Hematopoietic stem cell transplantation for adult Philadelphia-negative acute lymphoblastic leukemia in the first complete remission in the era of minimal residual disease. Curr Oncol Rep. 2018; 20:36. [Epub]. DOI: 10.1007/s11912-018-0679-9. PMID: 29577208.
Article6. Beatty PG, Clift RA, Mickelson EM, Nisperos BB, Flournoy N, Martin PJ, Sanders JE, Stewart P, Buckner CD, Storb R, Thomas DE, Hansen JA. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985; 313:765–771. DOI: 10.1056/NEJM198509263131301. PMID: 3897863.
Article7. Remberger M, Persson U, Hauzenberger D, Ringdén O. An association between human leucocyte antigen alleles and acute and chronic graft-versus-host disease after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2002; 119:751–759. DOI: 10.1046/j.1365-2141.2002.03924.x. PMID: 12437654.
Article8. Weisdorf D, Hakke R, Blazar B, Miller W, McGlave P, Ramsay N, Kersey J, Filipovich A. Risk factors for acute graft-versus-host disease in histocompatible donor bone marrow transplantation. Transplantation. 1991; 51:1197–1203. DOI: 10.1097/00007890-199106000-00010. PMID: 2048196.
Article9. Urbano-Ispizua A, Rozman C, Pimentel P, Solano C, de la Rubia J, Brunet S, Pérez-Oteyza J, Ferrá C, Zuazu J, Caballero D, Bargay J, Carvalhais A, Díez JL, Espigado I, Alegre A, Rovira M, Campilho F, Odriozola J, Sanz MA, Sierra J, García-Conde J, Montserrat E. Spanish Group for Allogeneic Peripheral Blood Transplantation (Grupo Español de Trasplante Hemopoyético) and Instituto Português de Oncologia-Porto. Risk factors for acute graft-versus-host disease in patients undergoing transplantation with CD34+ selected blood cells from HLA-identical siblings. Blood. 2002; 100:724–727. DOI: 10.1182/blood-2001-11-0057. PMID: 12091376.
Article10. Goulmy E, Schipper R, Pool J, Blokland E, Falkenburg JH, Vossen J, Gratwohl A, Vogelsang GB, van Houwelingen HC, van Rood JJ. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N Engl J Med. 1996; 334:281–285. DOI: 10.1056/NEJM199602013340501. PMID: 8532022.
Article11. Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, Hegland J, Kamani N, Kernan NA, King R, Ratanatharathorn V, Weisdorf D, Confer DL. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001; 98:2043–2051. DOI: 10.1182/blood.V98.7.2043. PMID: 11567988.
Article12. Cutler C, Giri S, Jeyapalan S, Paniagua D, Viswanathan A, Antin JH. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001; 19:3685–3691. DOI: 10.1200/JCO.2001.19.16.3685. PMID: 11504750.
Article13. Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, Urbano-Ispizua A, Pavletic SZ, Haagenson MD, Zhang MJ, Antin JH, Bolwell BJ, Bredeson C, Cahn JY, Cairo M, Gale RP, Gupta V, Lee SJ, Litzow M, Weisdorf DJ, Horowitz MM, Hahn T. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012; 119:296–307. DOI: 10.1182/blood-2011-06-364265. PMID: 22010102. PMCID: 3251233.
Article14. Wermke M, Maiwald S, Schmelz R, Thiede C, Schetelig J, Ehninger G, Bornhäuser M, Wassmuth R. Genetic variations of interleukin-23R (1143A>G) and BPI (A645G), but not of NOD2, are associated with acute graft-versus-host disease after allogeneic transplantation. Biol Blood Marrow Transplant. 2010; 16:1718–1727. DOI: 10.1016/j.bbmt.2010.06.001. PMID: 20541026.
Article15. Kim HT, Frederick D, Armand P, Andler E, Kao G, Cutler C, Koreth J, Alyea EP 3rd, Antin JH, Soiffer RJ, Ritz J, Ho VT. White blood cell recovery after allogeneic hematopoietic cell transplantation predicts clinical outcome. Am J Hematol. 2014; 89:591–597. DOI: 10.1002/ajh.23695. PMID: 24549932. PMCID: 4031274.
Article16. Lucchini G, Perales MA, Veys P. Immune reconstitution after cord blood transplantation: peculiarities, clinical implications and management strategies. Cytotherapy. 2015; 17:711–722. DOI: 10.1016/j.jcyt.2015.03.614. PMID: 25946726.
Article17. Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014; 124:344–353. DOI: 10.1182/blood-2014-02-514778. PMID: 24914142. PMCID: 4102707.
Article18. Lee S, Kim DW, Cho BS, Yoon JH, Shin SH, Yahng SA, Lee SE, Eom KS, Kim YJ, Chung NG, Kim HJ, Min CK, Lee JW, Min WS, Park CW. Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2012; 26:2367–2374. DOI: 10.1038/leu.2012.164. PMID: 22705993.
Article19. Park SS, Kim HJ, Min KI, Min GJ, Jeon YW, Yoon JH, Yahng SA, Shin SH, Lee SE, Cho BS, Eom KS, Kim YJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min WS. Prognostic prediction model for second allogeneic stem-cell transplantation in patients with relapsed acute myeloid leukemia: single-center report. Clin Lymphoma Myeloma Leuk. 2018; 18:e167–e182. DOI: 10.1016/j.clml.2018.02.009. PMID: 29519618.
Article20. Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016; 7:12150. [Epub]. DOI: 10.1038/ncomms12150. PMID: 27381735. PMCID: 4935811.
Article21. Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005; 106:2912–2919. DOI: 10.1182/blood-2005-05-2004. PMID: 15994282. PMCID: 1895304.
Article22. Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, Chanswangphuwana C, Efebera YA, Holler E, Litzow M, Ordemann R, Qayed M, Renteria AS, Reshef R, Wölfl M, Chen YB, Goldstein S, Jagasia M, Locatelli F, Mielke S, Porter D, Schechter T, Shekhovtsova Z, Ferrara JL, Levine JE. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the mount sinai acute GVHD international consortium. Biol Blood Marrow Transplant. 2016; 22:4–10. DOI: 10.1016/j.bbmt.2015.09.001. PMID: 26386318. PMCID: 4706482.
Article23. Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8− T cells in mice and humans. J Exp Med. 1994; 180:1097–1106. DOI: 10.1084/jem.180.3.1097. PMID: 7520467. PMCID: 2191643.
Article24. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007; 25:297–336. DOI: 10.1146/annurev.immunol.25.022106.141711. PMID: 17150027.
Article25. Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013; 13:101–117. DOI: 10.1038/nri3369. PMID: 23334244.
Article26. Haraguchi K, Takahashi T, Hiruma K, Kanda Y, Tanaka Y, Ogawa S, Chiba S, Miura O, Sakamaki H, Hirai H. Recovery of Valpha24+ NKT cells after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004; 34:595–602. DOI: 10.1038/sj.bmt.1704582. PMID: 15300228.
Article27. Rubio MT, Moreira-Teixeira L, Bachy E, Bouillié M, Milpied P, Coman T, Suarez F, Marcais A, Sibon D, Buzyn A, Caillat-Zucman S, Cavazzana-Calvo M, Varet B, Dy M, Hermine O, Leite-de-Moraes M. Early posttransplantation donor-derived invariant natural killer T-cell recovery predicts the occurrence of acute graft-versus-host disease and overall survival. Blood. 2012; 120:2144–2154. DOI: 10.1182/blood-2012-01-404673. PMID: 22730537.
Article28. Beziat V, Nguyen S, Exley M, Achour A, Simon T, Chevallier P, Sirvent A, Vigouroux S, Debré P, Rio B, Vieillard V. French Minicord Study Group. Shaping of iNKT cell repertoire after unrelated cord blood transplantation. Clin Immunol. 2010; 135:364–373. DOI: 10.1016/j.clim.2010.01.010. PMID: 20153980.
Article29. de Lalla C, Rinaldi A, Montagna D, Azzimonti L, Bernardo ME, Sangalli LM, Paganoni AM, Maccario R, Di Cesare-Merlone A, Zecca M, Locatelli F, Dellabona P, Casorati G. Invariant NKT cell reconstitution in pediatric leukemia patients given HLA-haploidentical stem cell transplantation defines distinct CD4+ and CD4− subset dynamics and correlates with remission state. J Immunol. 2011; 186:4490–4499. DOI: 10.4049/jimmunol.1003748. PMID: 21357532.
Article30. Dellabona P, Casorati G, de Lalla C, Montagna D, Locatelli F. On the use of donor-derived iNKT cells for adoptive immunotherapy to prevent leukemia recurrence in pediatric recipients of HLA haploidentical HSCT for hematological malignancies. Clin Immunol. 2011; 140:152–159. DOI: 10.1016/j.clim.2010.11.015. PMID: 21185785.
Article31. Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010; 40:2969–2975. DOI: 10.1002/eji.201040895. PMID: 21061430. PMCID: 3277452.
Article32. Lee SE, Lim JY, Kim TW, Jeon YW, Yoon JH, Cho BS, Eom KS, Kim YJ, Kim HJ, Lee S, Cho SG, Kim DW, Lee JW, Min WS, Shin DM, Choi EY, Min CK. Matrix metalloproteinase-9 in monocytic myeloid-derived suppressor cells correlate with early infections and clinical outcomes in allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018; 24:32–42. DOI: 10.1016/j.bbmt.2017.08.017. PMID: 28844945.
Article33. Hongo D, Tang X, Baker J, Engleman EG, Strober S. Requirement for interactions of natural killer T cells and myeloid-derived suppressor cells for transplantation tolerance. Am J Transplant. 2014; 14:2467–2477. DOI: 10.1111/ajt.12914. PMID: 25311657. PMCID: 4205183.
Article34. Gebremeskel S, Clattenburg DR, Slauenwhite D, Lobert L, Johnston B. Natural killer T cell activation overcomes immunosuppression to enhance clearance of postsurgical breast cancer metastasis in mice. Oncoimmunology. 2015; 4. [Epub]. DOI: 10.1080/2162402x.2014.995562. PMID: 25949924. PMCID: 4404817.
Article35. De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, Gröne HJ, Platt FM, Zambon M, Cerundolo V. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008; 118:4036–4048. DOI: 10.1172/JCI36264. PMID: 19033672. PMCID: 2582442.
Article36. Guan Q, Blankstein AR, Anjos K, Synova O, Tulloch M, Giftakis A, Yang B, Lambert P, Peng Z, Cuvelier GD, Wall DA. Functional myeloid-derived suppressor cell subsets recover rapidly after allogeneic hematopoietic stem/progenitor cell transplantation. Biol Blood Marrow Transplant. 2015; 21:1205–1214. DOI: 10.1016/j.bbmt.2015.04.015. PMID: 25963921.
Article37. Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8− alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993; 178:1–16. DOI: 10.1084/jem.178.1.1. PMID: 8391057. PMCID: 2191070.
Article38. Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4-8− T cells. J Exp Med. 1994; 180:1171–1176. DOI: 10.1084/jem.180.3.1171. PMID: 8064234. PMCID: 2191638.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Ovarian-Relapse Sparing of the Marrow in a Patient with Acute T Cell Lymphoblastic Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation
- Disseminated Aspergillosis following Allogeneic Hematopoietic Stem Cell Transplantation in an Acute Leukemic Patient who was Previously Treated for Invasive Aspergillosis
- A Case Report of the Second de Novo Acute Myeloid Leukemia (AML) Following Allogeneic Stem Cell Transplantation in a Patient with the First AML
- Unusual isolated extramedullary relapse of acute lymphoblastic leukemia in the breast despite complete donor hematopoietic chimerism after allogeneic hematopoietic stem cell transplantation
- Pediatric Allogeneic Hematopoietic Stem Cell Transplantation in Korea: April 2000: The Korean Society of Pediatric Hematology-Oncology