Korean J Radiol.
2019 Jun;20(6):967-975. 10.3348/kjr.2018.0690.
Combination of Magnetic Resonance Spectroscopy and ¹¹C-Methionine Positron Emission Tomography for the Accurate Diagnosis of Non-Enhancing Supratentorial Glioma
- Affiliations
-
- 1Department of Neurologic Surgery, Huashan Hospital, Shanghai Medical College, Fudan University, Shanghai, China. ernestzdx@163.com
- 2PET Center, Huashan Hospital, Shanghai Medical College, Fudan University, Shanghai, China.
- KMID: 2447075
- DOI: http://doi.org/10.3348/kjr.2018.0690
Abstract
OBJECTIVE
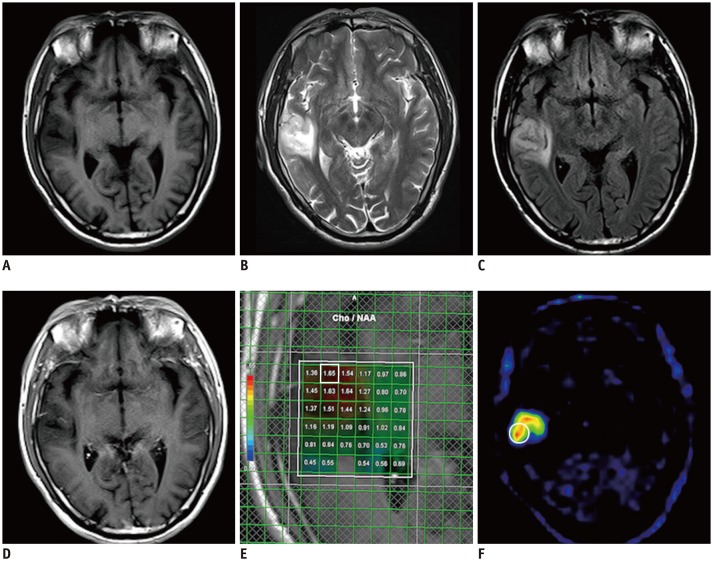
To evaluate whether the combination of magnetic resonance spectroscopy (MRS) and 11C-methionine positron emission tomography (11C-MET PET) could increase accurate diagnostic sensitivity for non-enhancing supratentorial gliomas.
MATERIALS AND METHODS
Between February 2012 and December 2017, 109 patients with non-enhanced supratentorial lesions on contrast-enhanced MRI were enrolled. Each patient underwent MRS and 11C-MET PET before treatment. A lesion was considered to be a glioma when either the MRS or 11C-MET PET results reached the diagnostic threshold. The radiological diagnosis was compared with the pathological diagnosis or medical diagnostic criteria.
RESULTS
The sensitivity and specificity were 60.0% and 50.0% for MRS and 75.8% and 50.0% for 11C-MET PET, respectively. Upon combining the two modalities, the sensitivity and specificity of the imaging-based diagnosis prior to surgery reached 89.5% and 42.9%, respectively. Statistically significant differences in the sensitivities were observed between the combined and individual approaches (MRS alone, 89.5% vs. 60.0%, p < 0.001; 11C-MET PET alone, 89.5% vs. 75.8%, p = 0.001). However, no significant differences in specificity were observed between the combined and individual modalities.
CONCLUSION
The combination of MRS and 11C-MET PET findings significantly increases accurate diagnostic sensitivity for non-enhancing supratentorial gliomas without significantly lowering the specificity. This finding suggests the potential of the combined MRS and 11C-MET PET approach in clinical applications.
Keyword
MeSH Terms
Figure
Reference
-
1. Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014; 16(Suppl 4):iv1–iv63. PMID: 25304271.
Article2. Kondziolka D, Lunsford LD, Martinez AJ. Unreliability of contemporary neurodiagnostic imaging in evaluating suspected adult supratentorial (low-grade) astrocytoma. J Neurosurg. 1993; 79:533–536. PMID: 8410222.
Article3. Fouke SJ, Benzinger T, Gibson D, Ryken TC, Kalkanis SN, Olson JJ. The role of imaging in the management of adults with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2015; 125:457–479. PMID: 26530262.4. Wang W, Hu Y, Lu P, Li Y, Chen Y, Tian M, et al. Evaluation of the diagnostic performance of magnetic resonance spectroscopy in brain tumors: a systematic review and meta-analysis. PloS One. 2014; 9:e112577. PMID: 25393009.
Article5. Martinez-Bisbal MC, Celda B. Proton magnetic resonance spectroscopy imaging in the study of human brain cancer. Q J Nucl Med Mol Imaging. 2009; 53:618–630. PMID: 20016453.6. Ha DH, Choi S, Oh JY, Yoon SK, Kang MJ, Kim KU. Application of 31P MR spectroscopy to the brain tumors. Korean J Radiol. 2013; 14:477–486. PMID: 23690717.7. Guo J, Yao C, Chen H, Zhuang D, Tang W, Ren G, et al. The relationship between Cho/NAA and glioma metabolism: implementation for margin delineation of cerebral gliomas. Acta Neurochir (Wien). 2012; 154:1361–1370. discussion 1370. PMID: 22729482.
Article8. Herholz K, Hölzer T, Bauer B, Schröder R, Voges J, Ernestus RI, et al. 11C-methionine pet for differential diagnosis of low-grade gliomas. Neurology. 1998; 50:1316–1322. PMID: 9595980.
Article9. Demetriades AK, Almeida AC, Bhangoo RS, Barrington SF. Applications of positron emission tomography in neuro-oncology: a clinical approach. Surgeon. 2014; 12:148–157. PMID: 24629841.
Article10. Glaudemans AW, Enting RH, Heesters MA, Dierckx RA, van Rheenen RW, Walenkamp AM, et al. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging. 2013; 40:615–635. PMID: 23232505.
Article11. Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M, et al. Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res. 2004; 10:7163–7170. PMID: 15534088.12. Kato T, Shinoda J, Nakayama N, Miwa K, Okumura A, Yano H, et al. Metabolic assessment of gliomas using 11C-methionine, [18F] fluorodeoxyglucose, and 11C-choline positron-emission tomography. AJNR Am J Neuroradiol. 2008; 29:1176–1182. PMID: 18388218.13. Ribom D, Eriksson A, Hartman M, Engler H, Nilsson A, Långström B, et al. Positron emission tomography (11) C-methionine and survival in patients with low-grade gliomas. Cancer. 2001; 92:1541–1549. PMID: 11745233.14. Tsuyuguchi N, Sunada I, Iwai Y, Yamanaka K, Tanaka K, Takami T, et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J Neurosurg. 2003; 98:1056–1064. PMID: 12744366.
Article15. Shishido H, Kawai N, Miyake K, Yamamoto Y, Nishiyama Y, Tamiya T. Diagnostic value of 11C-methionine (MET) and 18F-fluorothymidine (FLT) positron emission tomography in recurrent high-grade gliomas; differentiation from treatment-induced tissue necrosis. Cancers (Basel). 2012; 4:244–256. PMID: 24213238.
Article16. Pirotte B, Goldman S, Dewitte O, Massager N, Wikler D, Lefranc F, et al. Integrated positron emission tomography and magnetic resonance imaging-guided resection of brain tumors: a report of 103 consecutive procedures. J Neurosurg. 2006; 104:238–253. PMID: 16509498.
Article17. Matsuo M, Miwa K, Tanaka O, Shinoda J, Nishibori H, Tsuge Y, et al. Impact of [11C]methionine positron emission tomography for target definition of glioblastoma multiforme in radiation therapy planning. Int J Radiat Oncol Biol Phys. 2012; 82:83–89. PMID: 21095072.
Article18. Grosu AL, Weber WA, Riedel E, Jeremic B, Nieder C, Franz M, et al. L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys. 2005; 63:64–74. PMID: 16111573.
Article19. Mosskin M, Bergström M, Collins VP, Ehrin E, Eriksson L, von Holst H, et al. Positron emission tomography with 11C-methionine of intracranial tumours compared with histology of multiple biopsies. Acta Radiol Suppl. 1986; 369:157–160. PMID: 2980438.
Article20. Kaschten B, Stevenaert A, Sadzot B, Deprez M, Degueldre C, Del Fiore G, et al. Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J Nucl Med. 1998; 39:778–785. PMID: 9591574.21. Bulik M, Jancalek R, Vanicek J, Skoch A, Mechl M. Potential of MR spectroscopy for assessment of glioma grading. Clin Neurol Neurosurg. 2013; 115:146–153. PMID: 23237636.
Article22. Narayana A, Chang J, Thakur S, Huang W, Karimi S, Hou B, et al. Use of MR spectroscopy and functional imaging in the treatment planning of gliomas. Br J Radiol. 2007; 80:347–354. PMID: 17068012.
Article23. Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003; 24:1989–1998. PMID: 14625221.24. Yamasaki F, Takayasu T, Nosaka R, Amatya VJ, Doskaliyev A, Akiyama Y, et al. Magnetic resonance spectroscopy detection of high lipid levels in intraaxial tumors without central necrosis: a characteristic of malignant lymphoma. J Neurosurg. 2015; 122:1370–1379. PMID: 25748300.
Article25. Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014; 270:658–679. PMID: 24568703.
Article26. Baysal T, Ozisik HI, Karlidag R, Sarac K, Baysal O, Dusak A, et al. Proton MRS in Behcet's disease with and without neurological findings. Neuroradiology. 2003; 45:860–864. PMID: 14593444.27. Reijneveld JC, Sitskoorn MM, Klein M, Nuyen J, Taphoorn MJ. Cognitive status and quality of life in patients with suspected versus proven low-grade gliomas. Neurology. 2001; 56:618–623. PMID: 11245713.
Article28. Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgård G, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012; 308:1881–1888. PMID: 23099483.
Article29. Afra D, Osztie E, Sipos L, Vitanovics D. Preoperative history and postoperative survival of supratentorial low-grade astrocytomas. Br J Neurosurg. 1999; 13:299–305. PMID: 10562842.30. Potts MB, Smith JS, Molinaro AM, Berger MS. Natural history and surgical management of incidentally discovered low-grade gliomas. J Neurosurg. 2012; 116:365–372. PMID: 21999317.
Article31. Aghi MK, Nahed BV, Sloan AE, Ryken TC, Kalkanis SN, Olson JJ. The role of surgery in the management of patients with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2015; 125:503–530. PMID: 26530265.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Positron Emission Tomography-CT, CT, and MR Imaging Findings of Tumor-Mimicking Organized Hematoma in the Maxillary Sinus: Two Case Reports
- A Case of Sacroiliitis Diagnosed with Positron-Emission Tomography with Normal Magnetic Resonance Imaging Finding
- Current Radiopharmaceuticals for Positron Emission Tomography of Brain Tumors
- A Case of Basal Ganglia Germinoma Presenting Only with Cerebral Hemiatrophy Diagnosed by Using 11C-Methionine Positron Emission Tomography
- Quantitative Feasibility Evaluation of ¹¹C-Methionine Positron Emission Tomography Images in Gamma Knife Radiosurgery : Phantom-Based Study and Clinical Application