Nutr Res Pract.
2018 Jun;12(3):191-198. 10.4162/nrp.2018.12.3.191.
Comparison of the effect of three licorice varieties on cognitive improvement via an amelioration of neuroinflammation in lipopolysaccharide-induced mice
- Affiliations
-
- 1Department of Food Science and Nutrition, Pusan National University, 2 Busandaehak-ro 63 beon-gil, Geumjeong-gu, Busan 46241, Korea. ejcho@pusan.ac.kr
- 2Department of Herbal Crop Research, NIHHS, RDA, Chungbuk 27709, Korea.
- KMID: 2442292
- DOI: http://doi.org/10.4162/nrp.2018.12.3.191
Abstract
- BACKGROUND/OBJECTIVES
Neuroinflammation plays critical role in neurodegenerative disorders, such as Alzheimer's disease (AD). We investigated the effect of three licorice varieties, Glycyrhiza uralensis, G. glabra, and Shinwongam (SW) on a mouse model of inflammation-induced memory and cognitive deficit.
MATERIALS/METHODS
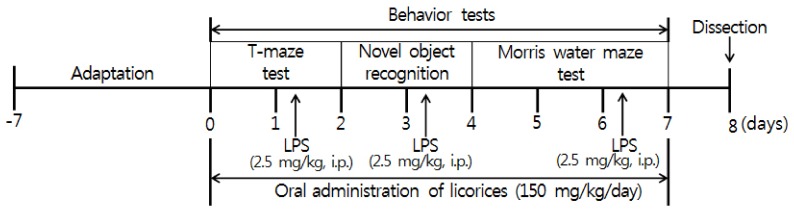
C57BL/6 mice were injected with lipopolysaccharide (LPS; 2.5 mg/kg, intraperitoneally) and orally administrated G. uralensis, G. glabra, and SW extract (150 mg/kg/day). SW, a new species of licorice in Korea, was combined with G. uralensis and G. glabra. Behavioral tests, including the T-maze, novel object recognition and Morris water maze, were carried out to assess learning and memory. In addition, the expressions of inflammation-related proteins in brain tissue were measured by western blotting.
RESULTS
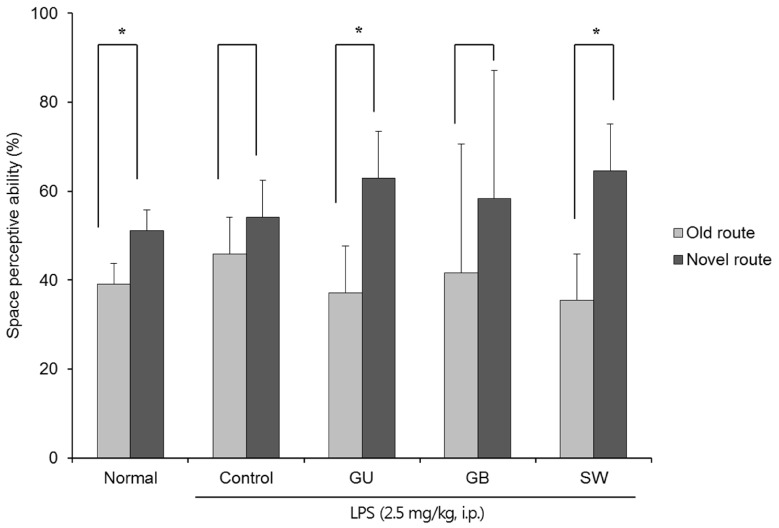
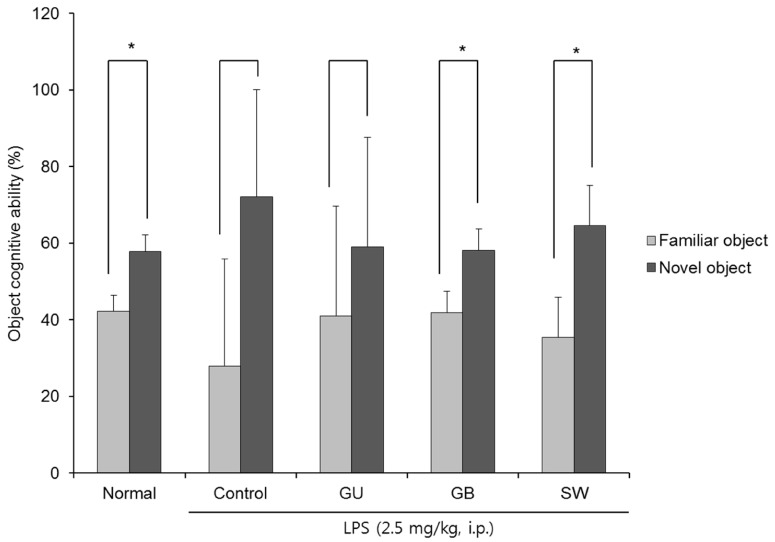
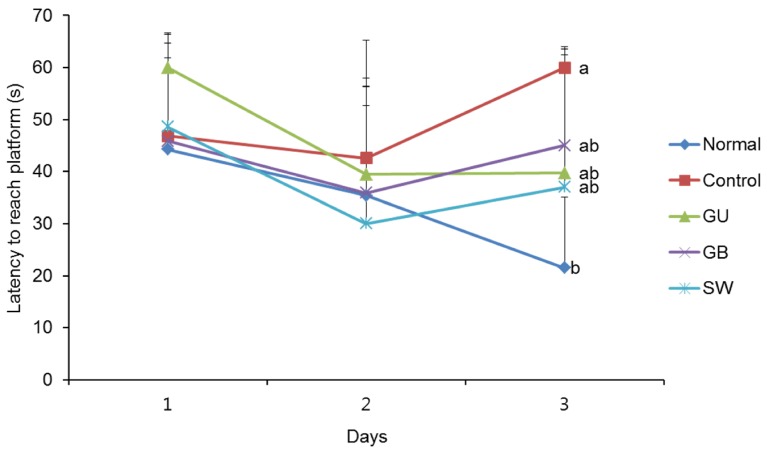
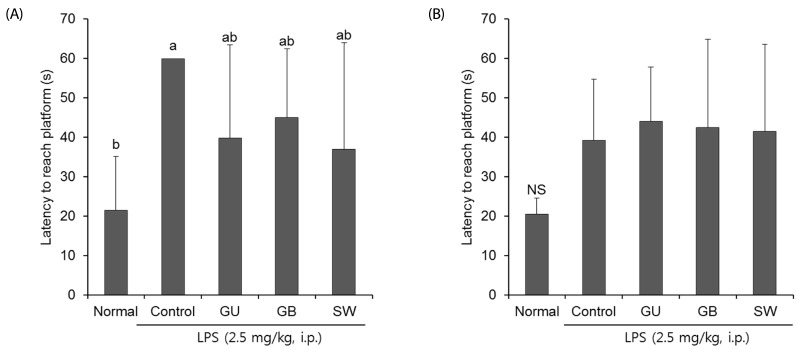
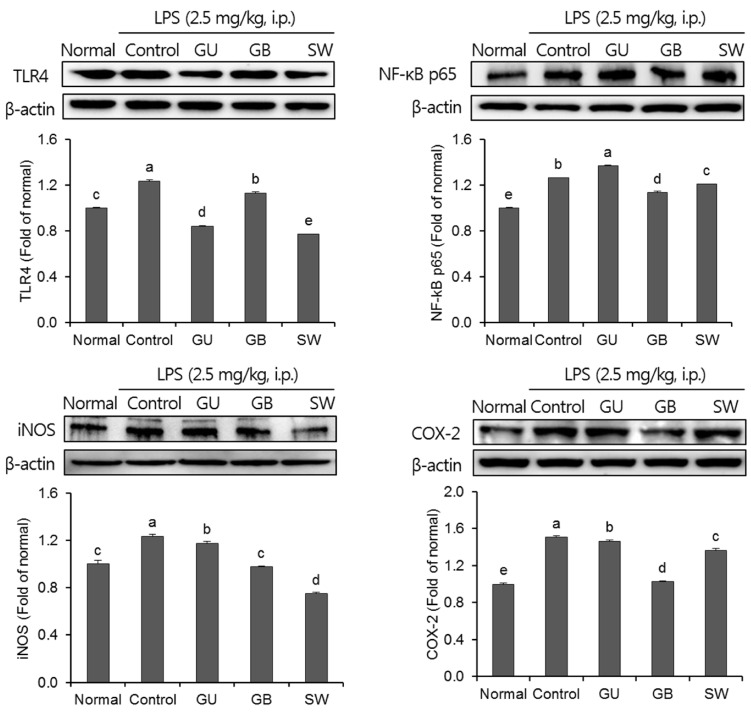
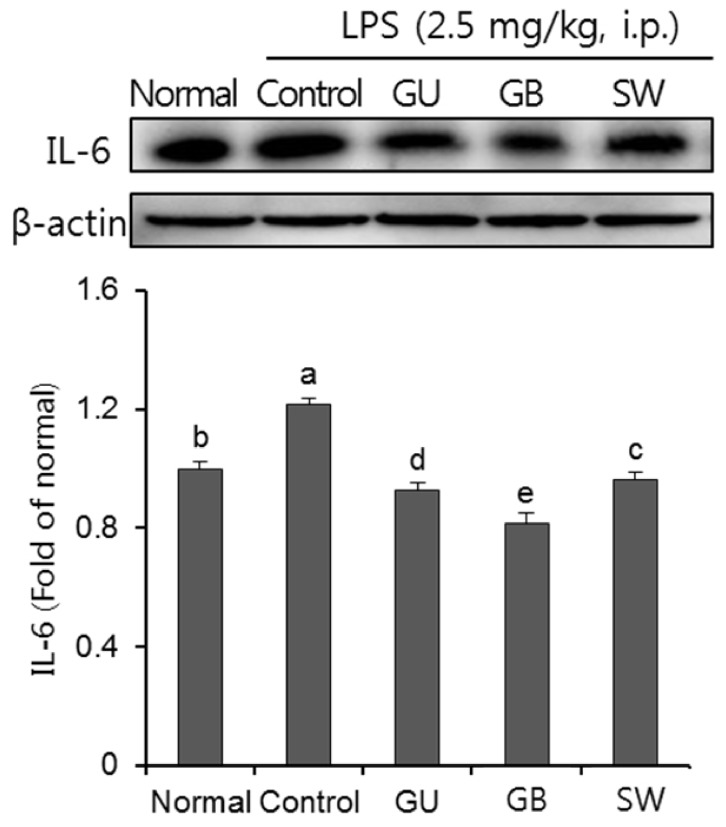
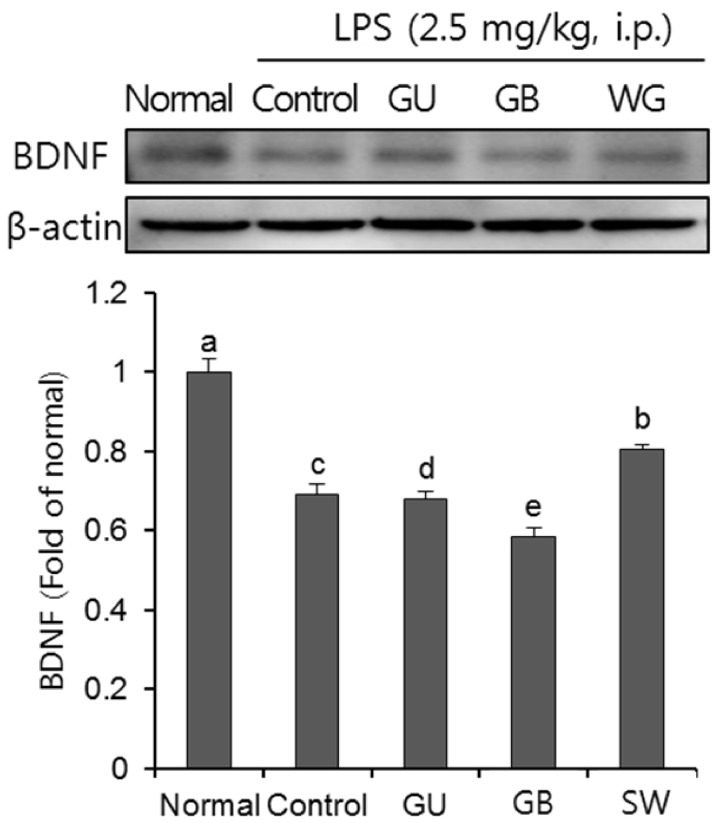
There was a significant decrease in spatial and objective recognition memory in LPS-induced cognitive impairment group, as measured by the T-maze and novel object recognition test; however, the administration of licorice ameliorated these deficits. In addition, licorice-treated groups exhibited improved learning and memory ability in the Morris water maze. Furthermore, LPS-injected mice had up-regulated pro-inflammatory proteins, such as inducible nitric oxide synthase (iNOS), cyclooxygenase-2, interleukin-6, via activation of toll like receptor 4 (TLR4) and nuclear factor-kappa B (NFκB) pathways in the brain. However, these were attenuated by following administration of the three licorice varieties. Interestingly, the SW-administered group showed greater inhibition of iNOS and TLR4 when compared with the other licorice varieties. Furthermore, there was a significant increase in the expression of brain-derived neurotrophic factor (BDNF) in the brain of LPS-induced cognitively impaired mice that were administered licorice, with the greatest effect following SW treatment.
CONCLUSIONS
The three licorice varieties ameliorated the inflammation-induced cognitive dysfunction by down-regulating inflammatory proteins and up-regulating BDNF. These results suggest that licorice, in particular SW, could be potential therapeutic agents against cognitive impairment.
MeSH Terms
-
Alzheimer Disease
Animals
Behavior Rating Scale
Blotting, Western
Brain
Brain-Derived Neurotrophic Factor
Cognition Disorders
Cyclooxygenase 2
Glycyrrhiza uralensis
Glycyrrhiza*
Inflammation
Interleukin-6
Korea
Learning
Memory
Mice*
Neurodegenerative Diseases
Nitric Oxide Synthase Type II
Toll-Like Receptor 4
Water
Brain-Derived Neurotrophic Factor
Cyclooxygenase 2
Interleukin-6
Nitric Oxide Synthase Type II
Toll-Like Receptor 4
Water
Figure
Reference
-
1. Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007; 3:186–191. PMID: 19595937.
Article2. Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. 1999; 45:358–368. PMID: 10072051.
Article3. Butterfield DA. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic Res. 2002; 36:1307–1313. PMID: 12607822.4. Canevari L, Abramov AY, Duchen MR. Toxicity of amyloid beta peptide: tales of calcium, mitochondria, and oxidative stress. Neurochem Res. 2004; 29:637–650. PMID: 15038611.5. Butterfield DA, Griffin S, Munch G, Pasinetti GM. Amyloid beta-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer's disease brain exists. J Alzheimers Dis. 2002; 4:193–201. PMID: 12226538.6. DeLegge MH, Smoke A. Neurodegeneration and inflammation. Nutr Clin Pract. 2008; 23:35–41. PMID: 18203962.
Article7. Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009; 137:47–59. PMID: 19345186.
Article8. Lukiw WJ. Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer's disease. Front Microbiol. 2016; 7:1544. PMID: 27725817.
Article9. Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007; 55:453–462. PMID: 17203472.
Article10. Rosi S, Vazdarjanova A, Ramirez-Amaya V, Worley PF, Barnes CA, Wenk GL. Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience. 2006; 142:1303–1315. PMID: 16989956.
Article11. Hanke ML, Kielian T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci (Lond). 2011; 121:367–387. PMID: 21745188.
Article12. Café-Mendes CC, Garay-Malpartida HM, Malta MB, de Sá Lima L, Scavone C, Ferreira ZS, Markus RP, Marcourakis T. Chronic nicotine treatment decreases LPS signaling through NF-κB and TLR-4 modulation in the hippocampus. Neurosci Lett. 2017; 636:218–224. PMID: 27984197.
Article13. Ray B, Lahiri DK. Neuroinflammation in Alzheimer's disease: different molecular targets and potential therapeutic agents including curcumin. Curr Opin Pharmacol. 2009; 9:434–444. PMID: 19656726.
Article14. Jain NK, Patil CS, Kulkarni SK, Singh A. Modulatory role of cyclooxygenase inhibitors in aging- and scopolamine or lipopolysaccharide-induced cognitive dysfunction in mice. Behav Brain Res. 2002; 133:369–376. PMID: 12110471.
Article15. Liao WC, Lin YH, Chang TM, Huang WY. Identification of two licorice species, Glycyrrhiza uralensis and Glycyrrhiza glabra, based on separation and identification of their bioactive components. Food Chem. 2012; 132:2188–2193.
Article16. Hosseinzadeh H, Nassiri-Asl M. Pharmacological effects of Glycyrrhiza spp. and its bioactive constituents: update and review. Phytother Res. 2015; 29:1868–1886. PMID: 26462981.17. Yang R, Yuan BC, Ma YS, Zhou S, Liu Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm Biol. 2017; 55:5–18. PMID: 27650551.
Article18. Barfod L, Kemp K, Hansen M, Kharazmi A. Chalcones from Chinese liquorice inhibit proliferation of T cells and production of cytokines. Int Immunopharmacol. 2002; 2:545–555. PMID: 11962733.
Article19. Yo YT, Shieh GS, Hsu KF, Wu CL, Shiau AL. Licorice and licochalcone-A induce autophagy in LNCaP prostate cancer cells by suppression of Bcl-2 expression and the mTOR pathway. J Agric Food Chem. 2009; 57:8266–8273. PMID: 19711916.
Article20. Lee HK, Yang EJ, Kim JY, Song KS, Seong YH. Inhibitory effects of Glycyrrhizae radix and its active component, isoliquiritigenin, on Aβ(25-35)-induced neurotoxicity in cultured rat cortical neurons. Arch Pharm Res. 2012; 35:897–904. PMID: 22644857.
Article21. Nomura T, Fukai T. Phenolic constituents of licorice (Glycyrrhiza species). Fortschr Chem Org Naturst. 1998; 73:1–158. PMID: 9545874.
Article22. Ojha S, Javed H, Azimullah S, Abul Khair SB, Haque ME. Glycyrrhizic acid attenuates neuroinflammation and oxidative stress in rotenone model of Parkinson's disease. Neurotox Res. 2016; 29:275–287. PMID: 26607911.
Article23. Cherng JM, Lin HJ, Hung MS, Lin YR, Chan MH, Lin JC. Inhibition of nuclear factor κB is associated with neuroprotective effects of glycyrrhizic acid on glutamate-induced excitotoxicity in primary neurons. Eur J Pharmacol. 2006; 547:10–21. PMID: 16952351.
Article24. Song JH, Lee JW, Shim B, Lee CY, Choi S, Kang C, Sohn NW, Shin JW. Glycyrrhizin alleviates neuroinflammation and memory deficit induced by systemic lipopolysaccharide treatment in mice. Molecules. 2013; 18:15788–15803. PMID: 24352029.
Article25. Akman T, Guven M, Aras AB, Ozkan A, Sen HM, Okuyucu A, Kalkan Y, Sehitoglu I, Silan C, Cosar M. The neuroprotective effect of glycyrrhizic acid on an experimental model of focal cerebral ischemia in rats. Inflammation. 2015; 38:1581–1588. PMID: 25687639.
Article26. Guo J, Yang CX, Yang JJ, Yao Y. Glycyrrhizic acid ameliorates cognitive impairment in a rat model of vascular dementia associated with oxidative damage and inhibition of voltage-gated sodium channels. CNS Neurol Disord Drug Targets. 2016; 15:1001–1008. PMID: 27238153.27. National Institute of Horticultural and Herbal Science (KR). 2014 Annual Reports. Wanju: National Institute of Horticultural and Herbal Science;2014. p. 58–59.28. Wang X, Zhang H, Chen L, Shan L, Fan G, Gao X. Liquorice, a unique “guide drug” of traditional Chinese medicine: a review of its role in drug interactions. J Ethnopharmacol. 2013; 150:781–790. PMID: 24201019.
Article29. Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008; 5:37. PMID: 18759972.
Article30. Gu SM, Park MH, Hwang CJ, Song HS, Lee US, Han SB, Oh KW, Ham YW, Song MJ, Son DJ, Hong JT. Bee venom ameliorates lipopolysaccharide-induced memory loss by preventing NF-kappaB pathway. J Neuroinflammation. 2015; 12:124. PMID: 26112466.
Article31. Montgomery KC. A test of two explanations of spontaneous alternation. J Comp Physiol Psychol. 1952; 45:287–293. PMID: 14946277.
Article32. Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006; 1:1306–1311. PMID: 17406415.
Article33. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11:47–60. PMID: 6471907.
Article34. Montgomery KC. Exploratory behavior and its relation to spontaneous alternation in a series of maze exposures. J Comp Physiol Psychol. 1952; 45:50–57. PMID: 14907933.
Article35. Lee YJ, Choi DY, Choi IS, Kim KH, Kim YH, Kim HM, Lee K, Cho WG, Jung JK, Han SB, Han JY, Nam SY, Yun YW, Jeong JH, Oh KW, Hong JT. Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models. J Neuroinflammation. 2012; 9:35. PMID: 22339795.
Article36. Miwa M, Tsuboi M, Noguchi Y, Enokishima A, Nabeshima T, Hiramatsu M. Effects of betaine on lipopolysaccharide-induced memory impairment in mice and the involvement of GABA transporter 2. J Neuroinflammation. 2011; 8:153. PMID: 22053950.
Article37. Sparkman NL, Martin LA, Calvert WS, Boehm GW. Effects of intraperitoneal lipopolysaccharide on Morris maze performance in year-old and 2-month-old female C57BL/6J mice. Behav Brain Res. 2005; 159:145–151. PMID: 15795008.
Article38. Sharifzadeh M, Shamsa F, Shiran S, Karimfar MH, Miri AH, Jalalizadeh H, Gholizadeh S, Salar F, Tabrizian K. A time course analysis of systemic administration of aqueous licorice extract on spatial memory retention in rats. Planta Med. 2008; 74:485–490. PMID: 18404595.
Article39. Dhingra D, Parle M, Kulkarni SK. Memory enhancing activity of Glycyrrhiza glabra in mice. J Ethnopharmacol. 2004; 91:361–365. PMID: 15120462.
Article40. Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer's disease, role of cytokines. ScientificWorldJournal. 2012; 2012:756357. PMID: 22566778.
Article41. Borowski T, Kokkinidis L, Merali Z, Anisman H. Lipopolysaccharide, central in vivo biogenic amine variations, and anhedonia. Neuroreport. 1998; 9:3797–3801. PMID: 9875707.
Article42. Lukiw WJ, Bazan NG. Strong nuclear factor-kappaB-DNA binding parallels cyclooxygenase-2 gene transcription in aging and in sporadic Alzheimer's disease superior temporal lobe neocortex. J Neurosci Res. 1998; 53:583–592. PMID: 9726429.43. Thiyagarajan P, Chandrasekaran CV, Deepak HB, Agarwal A. Modulation of lipopolysaccharide-induced pro-inflammatory mediators by an extract of Glycyrrhiza glabra and its phytoconstituents. Inflammopharmacology. 2011; 19:235–241. PMID: 21328091.
Article44. Wu TY, Khor TO, Saw CL, Loh SC, Chen AI, Lim SS, Park JH, Cai L, Kong AN. Anti-inflammatory/Anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J. 2011; 13:1–13. PMID: 20967519.
Article45. Schröfelbauer B, Raffetseder J, Hauner M, Wolkerstorfer A, Ernst W, Szolar OH. Glycyrrhizin, the main active compound in liquorice, attenuates pro-inflammatory responses by interfering with membrane-dependent receptor signalling. Biochem J. 2009; 421:473–482. PMID: 19442240.
Article46. Yu JY, Ha JY, Kim KM, Jung YS, Jung JC, Oh S. Anti-Inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules. 2015; 20:13041–13054. PMID: 26205049.47. Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci U S A. 2008; 105:2711–2716. PMID: 18263738.
Article48. Saura CA, Valero J. The role of CREB signaling in Alzheimer's disease and other cognitive disorders. Rev Neurosci. 2011; 22:153–169. PMID: 21476939.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Microglial galectin-3 increases with aging in the mouse hippocampus
- Humulus japonicus attenuates LPS-and scopolamine-induced cognitive impairment in mice
- Coadministration of 6-Shogaol and Levodopa Alleviates Parkinson’s Disease-Related Pathology in Mice
- Lipocalin-2 Acts as a Neuroinflammatogen in Lipopolysaccharide-injected Mice
- Conditioned Medium Derived from Salidroside-Pretreated Mesenchymal Stem Cell Culture Ameliorates Mouse Lipopolysaccharide-Induced Cerebral Neuroinflammation-Histological and Immunohistochemical Study