Tuberc Respir Dis.
2019 Jan;82(1):71-80. 10.4046/trd.2018.0049.
Expression of Muscarinic Receptors and the Effect of Tiotropium Bromide in Aged Mouse Model of Chronic Asthma
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. youngkim@catholic.ac.kr
- KMID: 2441732
- DOI: http://doi.org/10.4046/trd.2018.0049
Abstract
- BACKGROUND
Efficacy and safety of tiotropium bromide, a muscarinic receptor antagonist, in treatment of asthma have been reported. However, its effect on airway remodeling in chronic asthma of the elderly has not been clearly verified. The objective of this study was to investigate the effect of tiotropium and expression of muscarinic receptors as its related mechanism in an aged mouse model of chronic asthma with airway remodeling.
METHODS
BALB/c female mice age 6 weeks, 9 and 15 months were sensitized and challenged with ovalbumin (OVA) for three months. Tiotropium bromide was administered during the challenge period. Airway hyperresponsiveness (AHR) and pulmonary inflammation were measured. Parameters of airway remodeling, and expression levels of M2 and M3 receptors were examined.
RESULTS
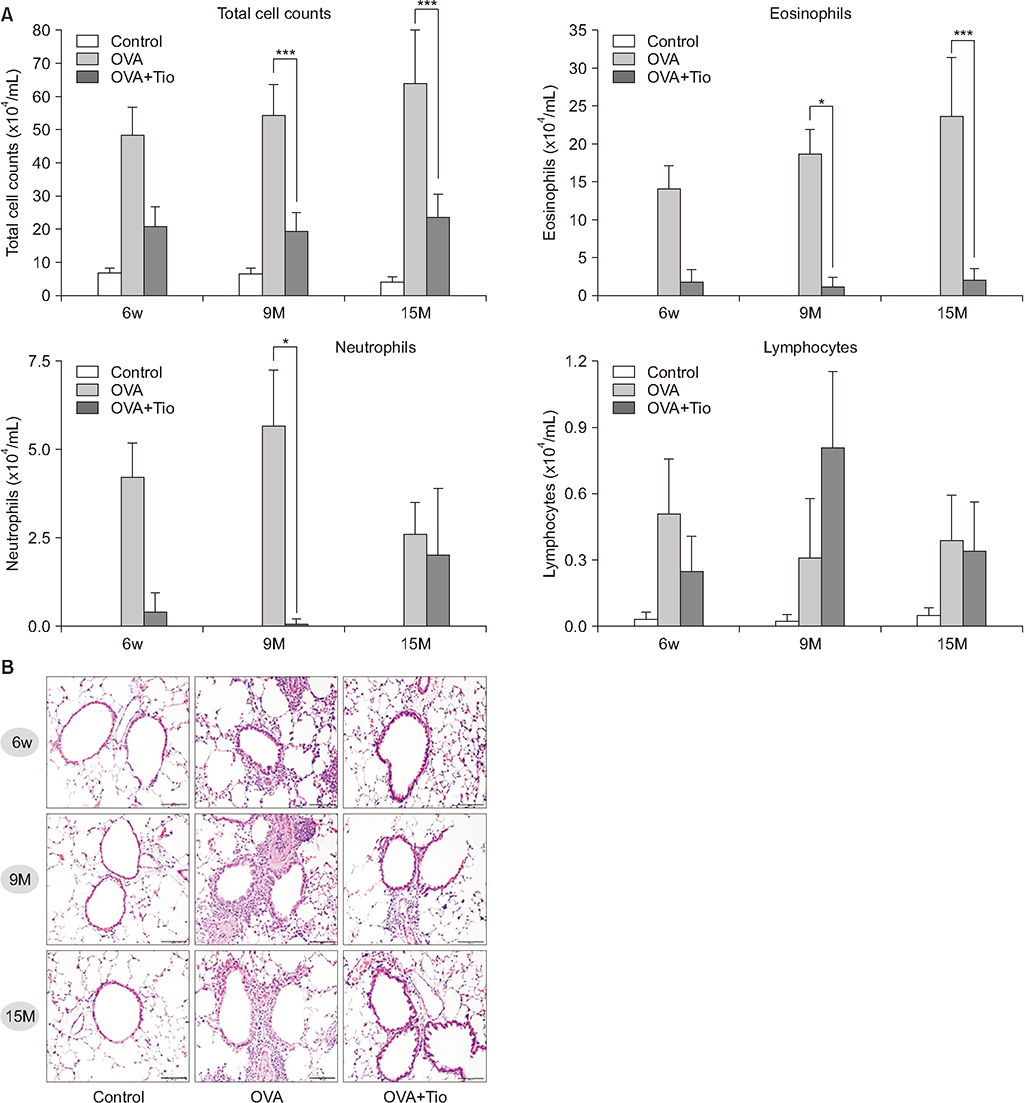
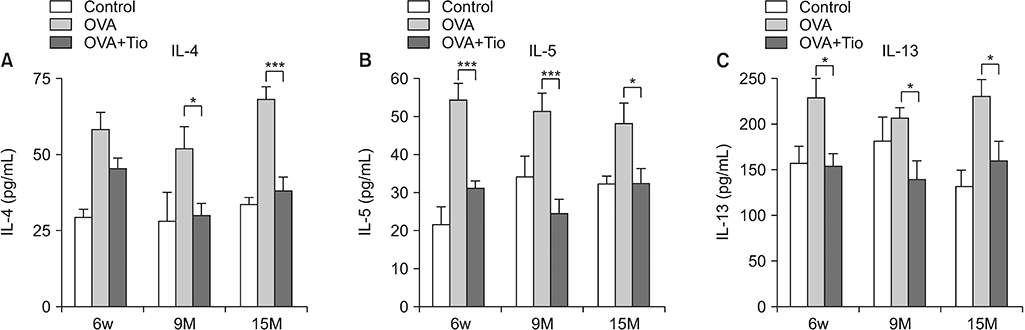
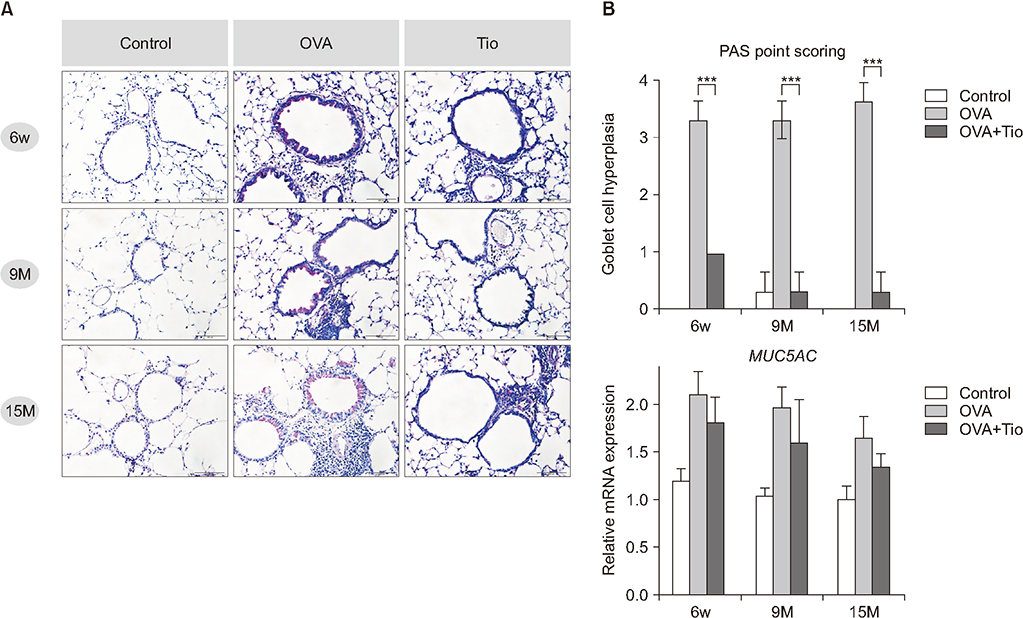
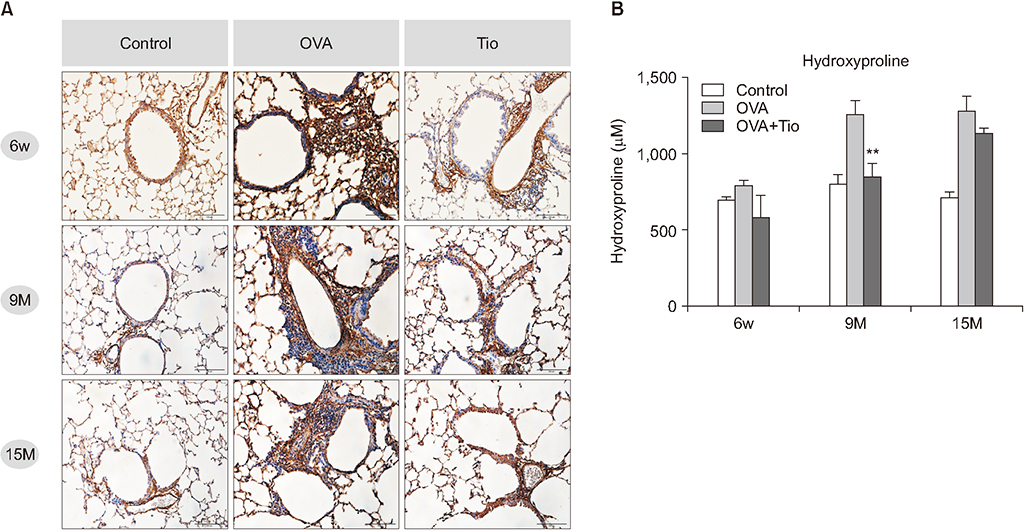
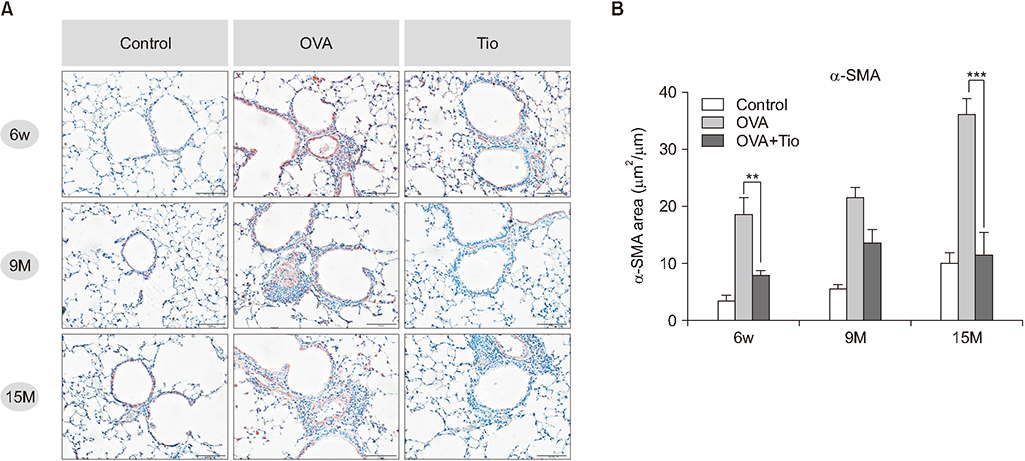
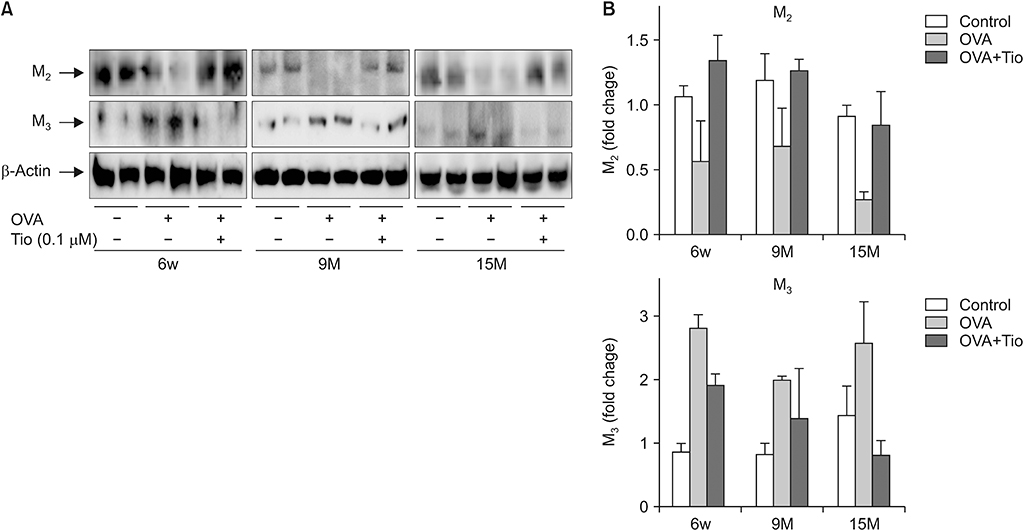
Total cell with eosinophils, increased in the OVA groups by age, was decreased significantly after treatment with tiotropium bromide, particularly in the age group of 15 months. AHR and levels of interleukin (IL)-4, IL-5, and IL-13 were decreased, after tiotropium administration. In old aged group of 9- and 15-months-treated groups, hydroxyproline contents and levels of α-smooth muscle actin were attenuated. Tiotropium enhanced the expression of M2 but decreased expression of M3 in all aged groups of OVA.
CONCLUSION
Tiotropium bromide had anti-inflammatory and anti-remodeling effects in an aged mouse model of chronic asthma. Its effects seemed to be partly mediated by modulating expression M3 and M2 muscarinic receptors. Tiotropium may be a beneficial treatment option for the elderly with airway remodeling of chronic asthma.
MeSH Terms
Figure
Reference
-
1. Cardona V, Guilarte M, Luengo O, Labrador-Horrillo M, Sala-Cunill A, Garriga T. Allergic diseases in the elderly. Clin Transl Allergy. 2011; 1:11.
Article2. Wuthrich B, Schmid-Grendelmeier P, Schindler C, Imboden M, Bircher A, Zemp E, et al. Prevalence of atopy and respiratory allergic diseases in the elderly SAPALDIA population. Int Arch Allergy Immunol. 2013; 162:143–148.
Article3. Park SY, Kim JH, Kim HJ, Seo B, Kwon OY, Chang HS, et al. High prevalence of asthma in elderly women: findings from a Korean national health database and adult asthma cohort. Allergy Asthma Immunol Res. 2018; 10:387–396.
Article4. Bellia V, Pedone C, Catalano F, Zito A, Davi E, Palange S, et al. Asthma in the elderly: mortality rate and associated risk factors for mortality. Chest. 2007; 132:1175–1182.5. De Martinis M, Sirufo MM, Ginaldi L. Allergy and aging: an old/new emerging health issue. Aging Dis. 2017; 8:162–175.
Article6. Vignola AM, Scichilone N, Bousquet J, Bonsignore G, Bellia V. Aging and asthma: pathophysiological mechanisms. Allergy. 2003; 58:165–175.
Article7. Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006; 7:73.
Article8. Rodrigo GJ, Castro-Rodriguez JA. Tiotropium for the treatment of adolescents with moderate to severe symptomatic asthma: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2015; 115:211–216.
Article9. Rodrigo GJ, Castro-Rodriguez JA. What is the role of tiotropium in asthma?: a systematic review with meta-analysis. Chest. 2015; 147:388–396.10. Cazzola M, Ora J, Rogliani P, Matera MG. Role of muscarinic antagonists in asthma therapy. Expert Rev Respir Med. 2017; 11:239–253.
Article11. Global Initiative for Asthma. 2016 GINA report, global strategy report for asthma management and prevention. Global Initiative for Asthma;2016.12. Kang JY, Lee SY, Rhee CK, Kim SJ, Kwon SS, Kim YK. Effect of aging on airway remodeling and muscarinic receptors in a murine acute asthma model. Clin Interv Aging. 2013; 8:1393–1403.
Article13. Kang JY, Rhee CK, Kim JS, Park CK, Kim SJ, Lee SH, et al. Effect of tiotropium bromide on airway remodeling in a chronic asthma model. Ann Allergy Asthma Immunol. 2012; 109:29–35.
Article14. Gosens R, Bos IS, Zaagsma J, Meurs H. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. Am J Respir Crit Care Med. 2005; 171:1096–1102.
Article15. Kim SR, Kim DI, Kang MR, Lee KS, Park SY, Jeong JS, et al. Endoplasmic reticulum stress influences bronchial asthma pathogenesis by modulating nuclear factor kappaB activation. J Allergy Clin Immunol. 2013; 132:1397–1408.16. Padrid P, Snook S, Finucane T, Shiue P, Cozzi P, Solway J, et al. Persistent airway hyperresponsiveness and histologic alterations after chronic antigen challenge in cats. Am J Respir Crit Care Med. 1995; 151:184–193.
Article17. Bosmans G, Shimizu Bassi G, Florens M, Gonzalez-Dominguez E, Matteoli G, Boeckxstaens GE. Cholinergic modulation of type 2 immune responses. Front Immunol. 2017; 8:1873.
Article18. Kistemaker LE, Gosens R. Acetylcholine beyond bronchoconstriction: roles in inflammation and remodeling. Trends Pharmacol Sci. 2015; 36:164–171.
Article19. Kistemaker LE, Bos ST, Mudde WM, Hylkema MN, Hiemstra PS, Wess J, et al. Muscarinic M(3) receptors contribute to allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol. 2014; 50:690–698.20. Bos IS, Gosens R, Zuidhof AB, Schaafsma D, Halayko AJ, Meurs H, et al. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J. 2007; 30:653–661.
Article21. Kistemaker LE, Hiemstra PS, Bos IS, Bouwman S, van den, Hylkema MN, et al. Tiotropium attenuates IL-13-induced goblet cell metaplasia of human airway epithelial cells. Thorax. 2015; 70:668–676.
Article22. Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010; 363:1715–1726.23. Hoshino M, Ohtawa J, Akitsu K. Effects of the addition of tiotropium on airway dimensions in symptomatic asthma. Allergy Asthma Proc. 2016; 37:147–153.
Article24. Busse PJ, Zhang TF, Srivastava K, Schofield B, Li XM. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin Exp Allergy. 2007; 37:1392–1403.
Article25. Busse PJ, Schofield B, Birmingham N, Yang N, Wen MC, Zhang T, et al. The traditional Chinese herbal formula ASHMI inhibits allergic lung inflammation in antigen-sensitized and antigen-challenged aged mice. Ann Allergy Asthma Immunol. 2010; 104:236–246.
Article26. Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000; 908:244–254.
Article27. Ventura MT, Scichilone N, Paganelli R, Minciullo PL, Patella V, Bonini M, et al. Allergic diseases in the elderly: biological characteristics and main immunological and non-immunological mechanisms. Clin Mol Allergy. 2017; 15:2.
Article28. Busse PJ, Mathur SK. Age-related changes in immune function: effect on airway inflammation. J Allergy Clin Immunol. 2010; 126:690–699.
Article29. Lee HK, Lim MY, Bok SM, Cho ES, Lee EM, Kim SW, et al. Age differences in cholinergic airway responsiveness in relation with muscarinic receptor subtypes. Life Sci. 2007; 81:204–209.
Article30. Ohta S, Oda N, Yokoe T, Tanaka A, Yamamoto Y, Watanabe Y, et al. Effect of tiotropium bromide on airway inflammation and remodelling in a mouse model of asthma. Clin Exp Allergy. 2010; 40:1266–1275.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Positioning of Long-Acting Muscarinic Antagonists in the Management of Asthma
- The role of tiotropium in the management of asthma
- Issues on Safety of Long-Acting Muscarinic Antagonist
- A Comparison of International Guidelines for Pediatric Asthma Pharmacotherapy
- Add-on Therapy for Symptomatic Asthma despite Long-Acting Beta-Agonists/Inhaled Corticosteroid