Positioning of Long-Acting Muscarinic Antagonists in the Management of Asthma
- Affiliations
-
- 1Department of Pulmonology, Martini Hospital, Groningen, The Netherlands.
- 2Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. hspark@ajou.ac.kr
- KMID: 2383985
- DOI: http://doi.org/10.4168/aair.2017.9.5.386
Abstract
- Despite a range of efficacious therapies for asthma, including inhaled corticosteroids (ICS) and long-acting β₂-agonists (LABA), a significant proportion of patients have poor asthma control and retain a risk of future worsening of their symptoms. Long-acting muscarinic antagonist (LAMA) bronchodilators offer a well-tolerated, efficacious, and cost-effective add-on to a patient's treatment. Of the LAMAs currently under investigation or available for the treatment of asthma, evidence from a comprehensive clinical trial program in adults and children shows that once-daily treatment with tiotropium provides benefits for patients with uncontrolled asthma despite the use of ICS and LABAs. Tiotropium is included in the Global Initiative for Asthma (GINA) strategy document as an add-on therapy option for patients at Step 4 or 5 with a history of asthma exacerbations. Tiotropium Respimat® has demonstrated safety and efficacy in patients with a range of disease severities, ages, and phenotypes. This review describes the evidence for the use of LAMA as add-on therapy for patients with asthma who remain uncontrolled despite the use of ICS and LABA treatments.
MeSH Terms
Figure
Cited by 2 articles
-
Characteristics of Adult Severe Refractory Asthma in Korea Analyzed From the Severe Asthma Registry
Min-Hye Kim, Sang-Heon Kim, So-Young Park, Ga-Young Ban, Joo-Hee Kim, Jae-Woo Jung, Ji Yong Moon, Woo-Jung Song, Hyouk-Soo Kwon, Jae-Woo Kwon, Jae Hyun Lee, Hye-Ryun Kang, Jong-Sook Park, Tae-Bum Kim, Heung-Woo Park, Kwang-Ha Yoo, Yeon-Mok Oh, Young-Il Koh, An-Soo Jang, Byung-Jae Lee, Young-Joo Cho, Sang-Heon Cho, Hae-Sim Park, Choon-Sik Park, Ho Joo Yoon, You Sook Cho
Allergy Asthma Immunol Res. 2019;11(1):43-54. doi: 10.4168/aair.2019.11.1.43.Add-on Tiotropium in Chinese Patients With Moderate Asthma: A Pooled Subgroup Analysis of MezzoTinA-Asthma 1 and 2
Jiangtao Lin, Huanying Wan, Jian Kang, Qianli Ma, Ping Chen, Meiling Jin, Haoyan Wang, Shuang Liu, Qinglin Hao, Yong Lin, Lin Su, Na Hu
Allergy Asthma Immunol Res. 2019;11(4):519-528. doi: 10.4168/aair.2019.11.4.519.
Reference
-
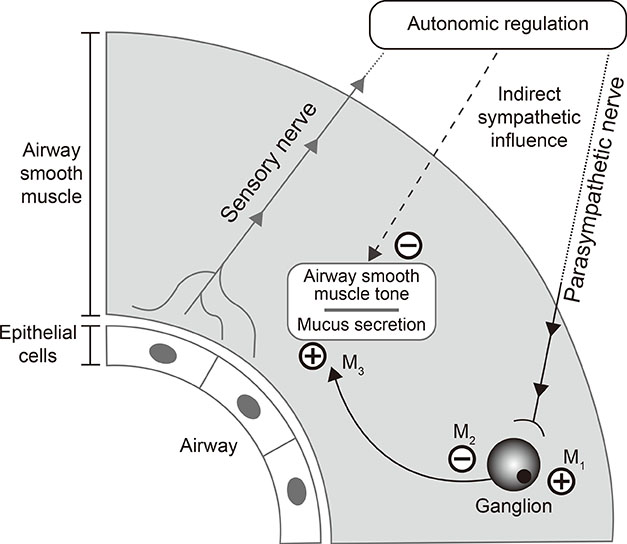
1. Global Asthma Network (NZ). The global asthma report: 2014. Auckland: Global Asthma Network;2014.2. World Health Organization. Asthma: fact sheet no.307. Geneva: World Health Organization;2013.3. Braman SS. The global burden of asthma. Chest. 2006; 130:4S–12S.4. Haughney J, Price D, Kaplan A, Chrystyn H, Horne R, May N, et al. Achieving asthma control in practice: understanding the reasons for poor control. Respir Med. 2008; 102:1681–1693.5. Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009; 39:193–202.6. Thompson PJ, Salvi S, Lin J, Cho YJ, Eng P, Abdul Manap R, et al. Insights, attitudes and perceptions about asthma and its treatment: findings from a multinational survey of patients from 8 Asia-Pacific countries and Hong Kong. Respirology. 2013; 18:957–967.7. Lai CK, De Guia TS, Kim YY, Kuo SH, Mukhopadhyay A, Soriano JB, et al. Asthma control in the Asia-Pacific region: the Asthma Insights and Reality in Asia-Pacific Study. J Allergy Clin Immunol. 2003; 111:263–268.8. Lai CK, Kim YY, Kuo SH, Spencer M, Williams AE. Cost of asthma in the Asia-Pacific region. Eur Respir Rev. 2006; 15:10–16.9. Global Initiative for Asthma. 2016 GINA report, global strategy for asthma management and prevention. [place unknown]: Global Initiative for Asthma;2016.10. Bateman ED, Harrison TW, Quirce S, Reddel HK, Buhl R, Humbert M, et al. Overall asthma control achieved with budesonide/formoterol maintenance and reliever therapy for patients on different treatment steps. Respir Res. 2011; 12:38.11. Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014; 24:14009.12. Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004; 170:836–844.13. Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006; 100:1139–1151.14. Park SY, Yoon SY, Shin B, Kwon HS, Kim TB, Moon HB, et al. Clinical factors affecting discrepant correlation between asthma control test score and pulmonary function. Allergy Asthma Immunol Res. 2015; 7:83–87.15. Demoly P, Annunziata K, Gubba E, Adamek L. Repeated cross-sectional survey of patient-reported asthma control in Europe in the past 5 years. Eur Respir Rev. 2012; 21:66–74.16. Accordini S, Corsico AG, Braggion M, Gerbase MW, Gislason D, Gulsvik A, et al. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol. 2013; 160:93–101.17. Price D, David-Wang A, Cho SH, Ho JC, Jeong JW, Liam CK, et al. Time for a new language for asthma control: results from REALISE Asia. J Asthma Allergy. 2015; 8:93–103.18. Price D, David-Wang A, Cho SH, Ho JC, Jeong JW, Liam CK, et al. Asthma in Asia: physician perspectives on control, inhaler use and patient communications. J Asthma. 2016; 53:761–769.19. Kolahian S, Gosens R. Cholinergic regulation of airway inflammation and remodelling. J Allergy (Cairo). 2012; 2012:681258.20. Barnes PJ. Neural mechanisms in asthma. Br Med Bull. 1992; 48:149–168.21. Price D, Fromer L, Kaplan A, van der Molen T, Román-Rodríguez M. Is there a rationale and role for long-acting anticholinergic bronchodilators in asthma? NPJ Prim Care Respir Med. 2014; 24:14023.22. Hashimoto A, Maeda H, Yokoyama M. Augmentation of parasympathetic nerve function in patients with extrinsic bronchial asthma--evaluation by coefficiency of variance of R-R interval with modified long-term ECG monitoring system. Kobe J Med Sci. 1996; 42:347–359.23. Gosens R, Bos IS, Zaagsma J, Meurs H. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. Am J Respir Crit Care Med. 2005; 171:1096–1102.24. Bos IS, Gosens R, Zuidhof AB, Schaafsma D, Halayko AJ, Meurs H, et al. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J. 2007; 30:653–661.25. Bateman ED, Rennard S, Barnes PJ, Dicpinigaitis PV, Gosens R, Gross NJ, et al. Alternative mechanisms for tiotropium. Pulm Pharmacol Ther. 2009; 22:533–542.26. Goyal M, Jaseja H, Verma N. Increased parasympathetic tone as the underlying cause of asthma: a hypothesis. Med Hypotheses. 2010; 74:661–664.27. Novelli F, Malagrinò L, Dente FL, Paggiaro P. Efficacy of anticholinergic drugs in asthma. Expert Rev Respir Med. 2012; 6:309–319.28. Belmonte KE. Cholinergic pathways in the lungs and anticholinergic therapy for chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005; 2:297–304.29. Barnes PJ. The pharmacological properties of tiotropium. Chest. 2000; 117:63S–66S.30. Koumis T, Samuel S. Tiotropium bromide: a new long-acting bronchodilator for the treatment of chronic obstructive pulmonary disease. Clin Ther. 2005; 27:377–392.31. Beeh KM, Moroni-Zentgraf P, Ablinger O, Hollaenderova Z, Unseld A, Engel M, et al. Tiotropium Respimat® in asthma: a double-blind, randomised, dose-ranging study in adult patients with moderate asthma. Respir Res. 2014; 15:61.32. Kistemaker LE, Gosens R. Acetylcholine beyond bronchoconstriction: roles in inflammation and remodeling. Trends Pharmacol Sci. 2015; 36:164–171.33. Kistemaker LE, Bos IS, Menzen MH, Maarsingh H, Meurs H, Gosens R. Combination therapy of tiotropium and ciclesonide attenuates airway inflammation and remodeling in a guinea pig model of chronic asthma. Respir Res. 2016; 17:13.34. O'Connor BJ, Towse LJ, Barnes PJ. Prolonged effect of tiotropium bromide on methacholine-induced bronchoconstriction in asthma. Am J Respir Crit Care Med. 1996; 154:876–880.35. Antoniu SA. Aclidinium bromide in experimental asthma. Expert Opin Investig Drugs. 2011; 20:871–873.36. Lee LA, Yang S, Kerwin E, Trivedi R, Edwards LD, Pascoe S. The effect of fluticasone furoate/umeclidinium in adult patients with asthma: a randomized, dose-ranging study. Respir Med. 2015; 109:54–62.37. Lee LA, Briggs A, Edwards LD, Yang S, Pascoe S. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015; 109:63–73.38. Blais CM, Davis BE, Cockcroft DW. Duration of bronchoprotection of the long-acting muscarinic antagonists tiotropium & glycopyrronium against methacholine-induced bronchoconstriction in mild asthmatics. Respir Med. 2016; 118:96–101.39. Kerstjens HA, Disse B, Schröder-Babo W, Bantje TA, Gahlemann M, Sigmund R, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011; 128:308–314.40. Bateman ED, Tashkin D, Siafakas N, Dahl R, Towse L, Massey D, et al. A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med. 2010; 104:1460–1472.41. Beeh KM, Kirsten AM, Dusser D, Sharma A, Cornelissen P, Sigmund R, et al. Pharmacodynamics and pharmacokinetics following once-daily and twice-daily dosing of tiotropium Respimat® in asthma using standardized sample-contamination avoidance. J Aerosol Med Pulm Drug Deliv. 2016; 29:406–415.42. Timmer W, Moroni-Zentgraf P, Cornelissen P, Unseld A, Pizzichini E, Buhl R. Once-daily tiotropium Respimat® 5 µg is an efficacious 24-h bronchodilator in adults with symptomatic asthma. Respir Med. 2015; 109:329–338.43. Vogelberg C, Moroni-Zentgraf P, Leonaviciute-Klimantaviciene M, Sigmund R, Hamelmann E, Engel M, et al. A randomised dose-ranging study of tiotropium Respimat® in children with symptomatic asthma despite inhaled corticosteroids. Respir Res. 2015; 16:20.44. Vogelberg C, Engel M, Moroni-Zentgraf P, Leonaviciute-Klimantaviciene M, Sigmund R, Downie J, et al. Tiotropium in asthmatic adolescents symptomatic despite inhaled corticosteroids: a randomised dose-ranging study. Respir Med. 2014; 108:1268–1276.45. Paggiaro P, Halpin DM, Buhl R, Engel M, Zubek VB, Blahova Z, et al. The effect of tiotropium in symptomatic asthma despite low- to medium-dose inhaled corticosteroids: a randomized controlled trial. J Allergy Clin Immunol Pract. 2016; 4:104–113.e2.46. Kerstjens HA, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015; 3:367–376.47. Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012; 367:1198–1207.48. Fajt ML, Wenzel SE. Development of new therapies for severe asthma. Allergy Asthma Immunol Res. 2017; 9:3–14.49. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014; 43:343–373.50. Kerstjens HA, Moroni-Zentgraf P, Tashkin DP, Dahl R, Paggiaro P, Vandewalker M, et al. Tiotropium improves lung function, exacerbation rate, and asthma control, independent of baseline characteristics including age, degree of airway obstruction, and allergic status. Respir Med. 2016; 117:198–206.51. Song WJ, Cho SH. Challenges in the management of asthma in the elderly. Allergy Asthma Immunol Res. 2015; 7:431–439.52. Trinh HK, Ban GY, Lee JH, Park HS. Leukotriene receptor antagonists for the treatment of asthma in elderly patients. Drugs Aging. 2016; 33:699–710.53. Ban GY, Ye YM, Lee Y, Kim JE, Nam YH, Lee SK, et al. Predictors of asthma control by stepwise treatment in elderly asthmatic patients. J Korean Med Sci. 2015; 30:1042–1047.54. Willson J, Bateman ED, Pavord I, Lloyd A, Krivasi T, Esser D. Cost effectiveness of tiotropium in patients with asthma poorly controlled on inhaled glucocorticosteroids and long-acting beta-agonists. Appl Health Econ Health Policy. 2014; 12:447–459.55. Echave M, Ojanguren ME, Elías I, de Andrés-Nogales F, Oyagüez I, Casado M, et al. Cost-effectiveness of tiotropium in the treatment of patients with asthma. Value Health. 2015; 18:A501–A502.56. Pawlik M, Walczak J, Pieniazek I. Economic evaluation of tiotropium administrated through the Respimat inhaler as add-on therapy in patients with uncontrolled severe asthma in Poland. Value Health. 2015; 18:A502.57. Silva Miguel L, Manaças M, Pinheiro B. Economic evaluation of tiotropium for severe persistent asthma in Portugal. Value Health. 2015; 18:A502.58. ClinicalTrials.gov (US). A Phase III parallel group study, comparing the efficacy, safety and tolerability of the fixed dose combination (FDC) of fluticasone furoate+umeclidinium bromide+vilanterol (FF/UMEC/VI) with the FDC of FF/VI in subjects with inadequately controlled asthma (NCT02924688). Bethesda (MD): ClinicalTrials.gov;2017.59. Yang S, Goyal N, Beerahee M, Trivedi R, Lee L, Pascoe S. Dose-response modelling of umeclidinium and fluticasone furoate/umeclidinium in asthma. Eur J Clin Pharmacol. 2015; 71:1051–1058.60. ClinicalTrials.gov (US). Chronic dosing cross-over study to assess the efficacy and safety of glycopyrronium (PT001) in adult subjects with intermittent asthma or mild to moderate persistent asthma (NCT02433834). Bethesda (MD): ClinicalTrials.gov;2016.61. ClinicalTrials.gov (US). A multicentre, randomised, double-blind, placebo-controlled, 2-way cross-over study to evaluate the efficacy and safety of CHF 5259 (glycopyrrolate bromide) pMDI on top of QVAR® pMDI for the treatment of patients with uncontrolled asthma on low-medium dose of inhaled corticosteroids (NCT02296411). Bethesda (MD): ClinicalTrials.gov;2016.62. ClinicalTrials.gov (US). The effect of glycopyrronium and indacaterol, as monotherapy and in combination, on the methacholine dose-response curve (NCT02953041). Bethesda (MD): ClinicalTrials.gov;2017.63. Ohta K, Ichinose M, Tohda Y, Engel M, Moroni-Zentgraf P, Kunimitsu S, et al. Long-term once-daily tiotropium Respimat® is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: a randomised, placebo-controlled study. PLoS One. 2015; 10:e0124109.64. Hamelmann E, Bateman ED, Vogelberg C, Szefler SJ, Vandewalker M, Moroni-Zentgraf P, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016; 138:441–450.e8.65. Hamelmann E, Bernstein JA, Vandewalker M, Moroni-Zentgraf P, Verri D, Unseld A, et al. A randomised controlled trial of tiotropium in adolescents with severe symptomatic asthma. Eur Respir J. 2017; 49:1601100.66. ClinicalTrials.gov (US). A randomised, double-blind, placebo-controlled, parallel-group trial to evaluate efficacy and safety of tiotropium inhalation solution (2.5 mcg and 5 mcg) delivered via Respimat® inhaler once daily in the evening over 48 weeks in children (6 to 11 years old) with moderate persistent asthma (NCT01634139). Bethesda (MD): ClinicalTrials.gov;2016.67. Szefler SJ, Murphy K, Harper T 3rd, Boner A, Laki I, Engel M, et al. A phase III randomized controlled trial of tiotropium add-on therapy in children with severe symptomatic asthma. J Allergy Clin Immunol. 2017; Forthcoming.68. ClinicalTrials.gov (US). A phase II/III, randomised, double-blind, placebo-controlled, parallel group trial to evaluate safety and efficacy of tiotropium inhalation solution (2.5 µg and 5 µg) administered once daily in the afternoon via Respimat® inhaler for 12 weeks in patients 1 to 5 years old with persistent asthma (NCT01634113). Bethesda (MD): ClinicalTrials.gov;2015.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Bronchodilators in Preventing Exacerbations of Chronic Obstructive Pulmonary Disease
- Issues on Safety of Long-Acting Muscarinic Antagonist
- Respiratory Review of 2011: Asthma
- Optimal Bronchodilation for COPD Patients: Are All Long-Acting β₂-Agonist/Long-Acting Muscarinic Antagonists the Same?
- The role of tiotropium in the management of asthma