Far-infrared radiation stimulates platelet-derived growth factor mediated skeletal muscle cell migration through extracellular matrix-integrin signaling
- Affiliations
-
- 1Department of Physiology, Chung-Ang University College of Medicine, Seoul 06974, Korea. akdongyi01@cau.ac.kr
- 2Department of Family Medicine, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul 06973, Korea. girlpower219@cau.ac.kr
- KMID: 2438088
- DOI: http://doi.org/10.4196/kjpp.2019.23.2.141
Abstract
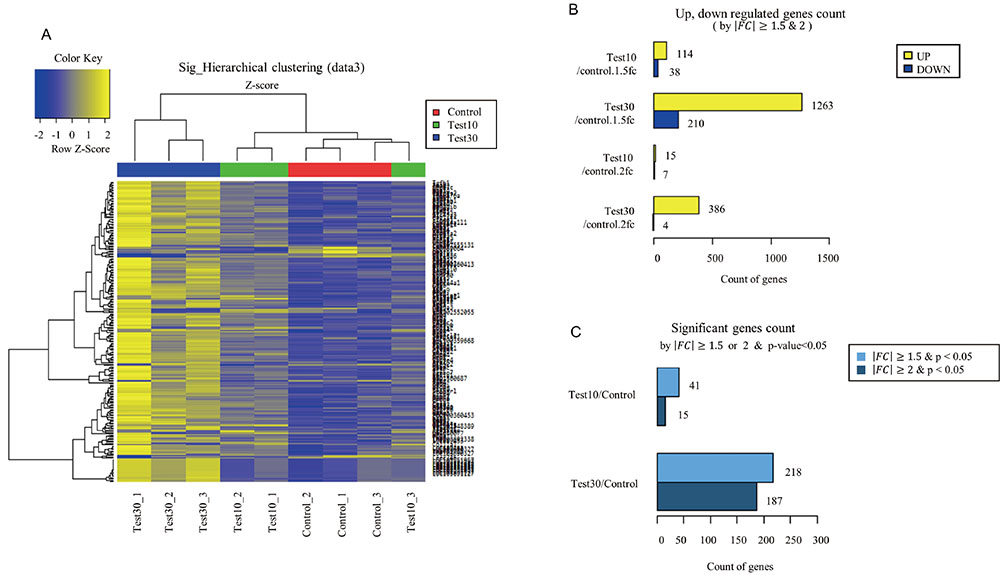
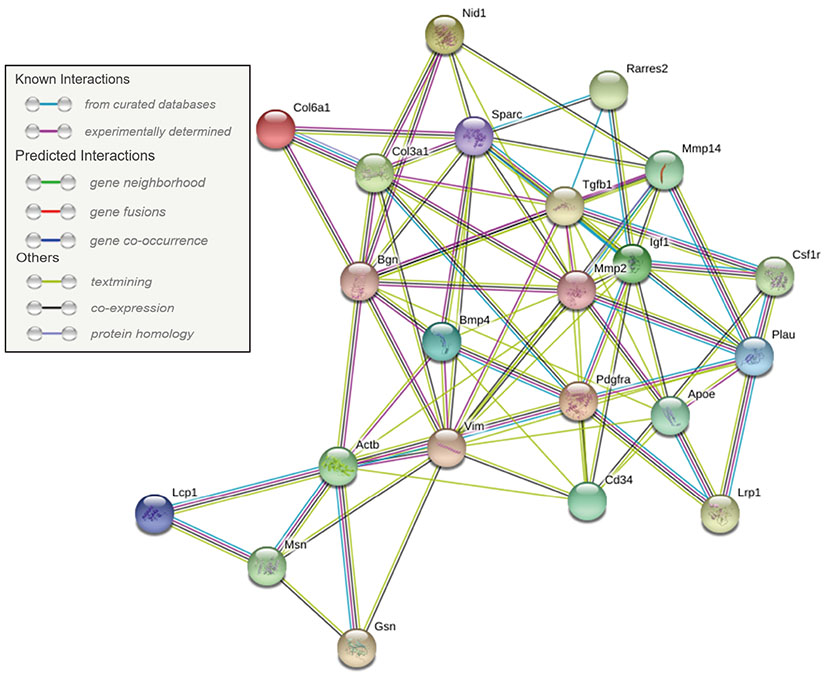
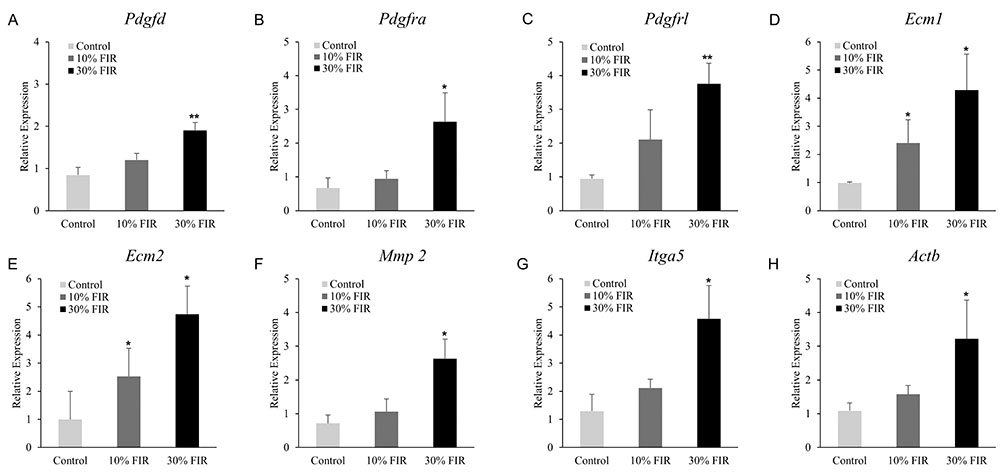
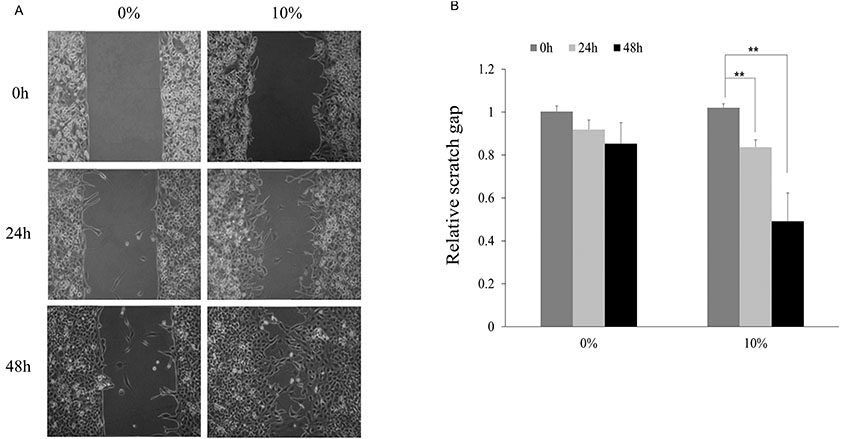
- Despite increased evidence of bio-activity following far-infrared (FIR) radiation, susceptibility of cell signaling to FIR radiation-induced homeostasis is poorly understood. To observe the effects of FIR radiation, FIR-radiated materials-coated fabric was put on experimental rats or applied to L6 cells, and microarray analysis, quantitative real-time polymerase chain reaction, and wound healing assays were performed. Microarray analysis revealed that messenger RNA expressions of rat muscle were stimulated by FIR radiation in a dose-dependent manner in amount of 10% and 30% materials-coated. In 30% group, 1,473 differentially expressed genes were identified (fold change [FC] > 1.5), and 218 genes were significantly regulated (FC > 1.5 and p < 0.05). Microarray analysis showed that extracellular matrix (ECM)-receptor interaction, focal adhesion, and cell migration-related pathways were significantly stimulated in rat muscle. ECM and platelet-derived growth factor (PDGF)-mediated cell migration-related genes were increased. And, results showed that the relative gene expression of actin beta was increased. FIR radiation also stimulated actin subunit and actin-related genes. We observed that wound healing was certainly promoted by FIR radiation over 48 h in L6 cells. Therefore, we suggest that FIR radiation can penetrate the body and stimulate PDGF-mediated cell migration through ECM-integrin signaling in rats.
Keyword
MeSH Terms
-
Actins
Animals
Cell Movement*
Extracellular Matrix
Focal Adhesions
Gene Expression
Homeostasis
Infrared Rays
Integrins
Microarray Analysis
Muscle, Skeletal*
Platelet-Derived Growth Factor*
Rats
Real-Time Polymerase Chain Reaction
RNA, Messenger
Wound Healing
Actins
Integrins
Platelet-Derived Growth Factor
RNA, Messenger
Figure
Cited by 2 articles
-
Far-infrared rays enhance mitochondrial biogenesis and
GLUT3 expression under low glucose conditions in rat skeletal muscle cells
Yelim Seo, Young-Won Kim, Donghee Lee, Donghyeon Kim, Kyoungseo Kim, Taewoo Kim, Changyeob Baek, Yerim Lee, Junhyeok Lee, Hosung Lee, Geonwoo Jang, Wonyeong Jeong, Junho Choi, Doegeun Hwang, Jung Soo Suh, Sun-Woo Kim, Hyoung Kyu Kim, Jin Han, Hyoweon Bang, Jung-Ha Kim, Tong Zhou, Jae-Hong Ko
Korean J Physiol Pharmacol. 2021;25(2):167-175. doi: 10.4196/kjpp.2021.25.2.167.Cardioprotection
via mitochondrial transplantation supports fatty acid metabolism in ischemia-reperfusion injured rat heart
Jehee Jang, Ki-Woon Kang, Young-Won Kim, Seohyun Jeong, Jaeyoon Park, Jihoon Park, Jisung Moon, Junghyun Jang, Seohyeon Kim, Sunghun Kim, Sungjoo Cho, Yurim Lee, Hyoung Kyu Kim, Jin Han, Eun-A Ko, Sung-Cherl Jung, Jung-Ha Kim, Jae-Hong Ko
Korean J Physiol Pharmacol. 2024;28(3):209-217. doi: 10.4196/kjpp.2024.28.3.209.
Reference
-
1. Yu SY, Chiu JH, Yang SD, Hsu YC, Lui WY, Wu CW. Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Photodermatol Photoimmunol Photomed. 2006; 22:78–86.
Article2. Beever R. Far-infrared saunas for treatment of cardiovascular risk factors: summary of published evidence. Can Fam Physician. 2009; 55:691–696.3. Adamskaya N, Dungel P, Mittermayr R, Hartinger J, Feichtinger G, Wassermann K, Redl H, van Griensven M. Light therapy by blue LED improves wound healing in an excision model in rats. Injury. 2011; 42:917–921.
Article4. Vatansever F, Hamblin MR. Far infrared radiation (FIR): its biological effects and medical applications. Photonics Lasers Med. 2012; 4:255–266.
Article5. Tsai SR, Hamblin MR. Biological effects and medical applications of infrared radiation. J Photochem Photobiol B. 2017; 170:197–207.
Article6. Inoué S, Kabaya M. Biological activities caused by far-infrared radiation. Int J Biometeorol. 1989; 33:145–150.
Article7. Rau CS, Yang JC, Jeng SF, Chen YC, Lin CJ, Wu CJ, Lu TH, Hsieh CH. Far-infrared radiation promotes angiogenesis in human microvascular endothelial cells via extracellular signal-regulated kinase activation. Photochem Photobiol. 2011; 87:441–446.
Article8. Tran TH, Mai HN, Shin EJ, Nam Y, Nguyen BT, Lee YJ, Jeong JH, Tran HY, Cho EH, Nah SY, Lei XG, Nabeshima T, Kim NH, Kim HC. Repeated exposure to far infrared ray attenuates acute restraint stress in mice via inhibition of JAK2/STAT3 signaling pathway by induction of glutathione peroxidase-1. Neurochem Int. 2016; 94:9–22.
Article9. Huang PH, Chen JW, Lin CP, Chen YH, Wang CH, Leu HB, Lin SJ. Far infra-red therapy promotes ischemia-induced angiogenesis in diabetic mice and restores high glucose-suppressed endothelial progenitor cell functions. Cardiovasc Diabetol. 2012; 11:99.
Article10. Yang CS, Yeh CH, Tung CL, Chen MY, Jiang CH, Yeh ML. Impact of far-infrared ray exposure on the mechanical properties of unwounded skin of rats. Exp Biol Med (Maywood). 2010; 235:952–956.
Article11. Shui S, Wang X, Chiang JY, Zheng L. Far-infrared therapy for cardiovascular, autoimmune, and other chronic health problems: a systematic review. Exp Biol Med (Maywood). 2015; 240:1257–1265.12. Hsu YH, Chen YC, Chen TH, Sue YM, Cheng TH, Chen JR, Chen CH. Far-infrared therapy induces the nuclear translocation of PLZF which inhibits VEGF-induced proliferation in human umbilical vein endothelial cells. PLoS One. 2012; 7:e30674.
Article13. Noponen PVA, Häkkinen K, Mero AA. Effects of far infrared heat on recovery in power athletes. J Athl Enhancement. 2015; 4:DOI: 10.4172/2324-9080.1000202.
Article14. Mai HN, Sharma N, Shin EJ, Nguyen BT, Nguyen PT, Jeong JH, Jang CG, Cho EH, Nah SY, Kim NH, Nabeshima T, Kim HC. Exposure to far-infrared rays attenuates methamphetamine-induced recognition memory impairment via modulation of the muscarinic M1 receptor, Nrf2, and PKC. Neurochem Int. 2018; 116:63–76.
Article15. Chang HY, Shih MH, Huang HC, Tsai SR, Juan HF, Lee SC. Middle infrared radiation induces G2/M cell cycle arrest in A549 lung cancer cells. PLoS One. 2013; 8:e54117.
Article16. Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003; 302:1704–1709.
Article17. Sheetz MP, Felsenfeld DP, Galbraith CG. Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 1998; 8:51–54.
Article18. Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002; 2:91–100.
Article19. Krawczyk C, Oliveira-dos-Santos A, Sasaki T, Griffiths E, Ohashi PS, Snapper S, Alt F, Penninger JM. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity. 2002; 16:331–343.
Article20. Blazevic T, Schwaiberger AV, Schreiner CE, Schachner D, Schaible AM, Grojer CS, Atanasov AG, Werz O, Dirsch VM, Heiss EH. 12/15-lipoxygenase contributes to platelet-derived growth factor-induced activation of signal transducer and activator of transcription 3. J Biol Chem. 2013; 288:35592–35603.21. Alvarez RH, Kantarjian HM, Cortes JE. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc. 2006; 81:1241–1257.
Article22. Yu J, Moon A, Kim HR. Both platelet-derived growth factor receptor (PDGFR)-alpha and PDGFR-beta promote murine fibroblast cell migration. Biochem Biophys Res Commun. 2001; 282:697–700.23. Robbins JR, McGuire PG, Wehrle-Haller B, Rogers SL. Diminished matrix metalloproteinase 2 (MMP-2) in ectomesenchyme-derived tissues of the Patch mutant mouse: regulation of MMP-2 by PDGF and effects on mesenchymal cell migration. Dev Biol. 1999; 212:255–263.
Article24. Xu J, Clark RA. Extracellular matrix alters PDGF regulation of fibroblast integrins. J Cell Biol. 1996; 132:239–249.
Article25. Zhang F, Hao F, An D, Zeng L, Wang Y, Xu X, Cui MZ. The matricellular protein Cyr61 is a key mediator of platelet-derived growth factor-induced cell migration. J Biol Chem. 2015; 290:8232–8242.
Article26. Li J, Kim YN, Bertics PJ. Platelet-derived growth factor-stimulated migration of murine fibroblasts is associated with epidermal growth factor receptor expression and tyrosine phosphorylation. J Biol Chem. 2000; 275:2951–2958.
Article27. Raines EW, Lane TF, Iruela-Arispe ML, Ross R, Sage EH. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci U S A. 1992; 89:1281–1285.
Article28. Yuan L, Santi M, Rushing EJ, Cornelison R, MacDonald TJ. ERK activation of p21 activated kinase-1 (Pak1) is critical for medulloblastoma cell migration. Clin Exp Metastasis. 2010; 27:481–491.
Article29. MacDonald TJ, Brown KM, LaFleur B, Peterson K, Lawlor C, Chen Y, Packer RJ, Cogen P, Stephan DA. Expression profiling of medulloblastoma: PDGFRA and the RAS/MAPK pathway as therapeutic targets for metastatic disease. Nat Genet. 2001; 29:143–152.
Article30. Borkham-Kamphorst E, van Roeyen CR, Ostendorf T, Floege J, Gressner AM, Weiskirchen R. Pro-fibrogenic potential of PDGF-D in liver fibrosis. J Hepatol. 2007; 46:1064–1074.
Article31. Veevers-Lowe J, Ball SG, Shuttleworth A, Kielty CM. Mesenchymal stem cell migration is regulated by fibronectin through α5β1-integrin-mediated activation of PDGFR-β and potentiation of growth factor signals. J Cell Sci. 2011; 124:1288–1300.
Article32. Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994; 372:786–791.
Article33. Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008; 9:344–356.
Article34. Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration--the actin connection. J Cell Sci. 2009; 122:199–206.35. Peterson EJ. The TCR ADAPts to integrin-mediated cell adhesion. Immunol Rev. 2003; 192:113–121.
Article36. Hsu YH, Lin YF, Chen CH, Chiu YJ, Chiu HW. Far infrared promotes wound healing through activation of Notch1 signaling. J Mol Med (Berl). 2017; 95:1203–1213.
Article37. Lee D, Kim YW, Kim JH, Yang M, Bae H, Lim I, Bang H, Go KC, Yang GW, Rho YH, Park HS, Park EH, Ko JH. Improvement characteristics of bio-active materials coated fabric on rat muscular mitochondria. Korean J Physiol Pharmacol. 2015; 19:283–289.
Article38. Lee SG, Lee CG, Wu HM, Oh CS, Chung SW, Kim SG. A load of mice to hypergravity causes AMPKα repression with liver injury, which is overcome by preconditioning loads via Nrf2. Sci Rep. 2015; 5:15643.
Article39. Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015; 43:D447–D452.
Article40. Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat. 2015; 11:2191–2208.
Article41. Zhu J, Clark RAF. Fibronectin at select sites binds multiple growth factors and enhances their activity: expansion of the collaborative ECM-GF paradigm. J Invest Dermatol. 2014; 134:895–901.
Article42. Laurent M, Martinerie C, Thibout H, Hoffman MP, Verrecchia F, Le Bouc Y, Mauviel A, Kleinman HK. NOVH increases MMP3 expression and cell migration in glioblastoma cells via a PDGFR-alphadependent mechanism. FASEB J. 2003; 17:1919–1921.43. Hardee JP, Puppa MJ, Fix DK, Gao S, Hetzler KL, Bateman TA, Carson JA. The effect of radiation dose on mouse skeletal muscle remodeling. Radiol Oncol. 2014; 48:247–256.
Article44. Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel alpha3 isoform. J Cell Biol. 1997; 137:685–701.45. Boraschi-Diaz I, Wang J, Mort JS, Komarova SV. Collagen type I as a ligand for receptor-mediated signaling. Front Phys. 2017; 5:DOI: 10.3389/fphy.2017.00012.
Article46. Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992; 70:389–399.
Article47. Duchek P, Somogyi K, Jékely G, Beccari S, Rørth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001; 107:17–26.
Article48. Le Clainche C, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. 2008; 88:489–513.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphological Analysis of Intimal Hyperplasia in Allografted Aorta of Rat
- Proliferative and Synthetic Responses of Airway Smooth Muscle in Asthma
- betaig-h3 triggers signaling pathways mediating adhesion and migration of vascular smooth muscle cells through alphavbeta5 integrin
- Adipose-Derived Stem Cell Coculturing Stimulates Integrin-Mediated Extracellular Matrix Adhesion of Melanocytes by Upregulating Growth Factors
- Expression of alpha3beta1 Integrin in ECV304 Endothelial Cells and Angiogenesis