Nutr Res Pract.
2016 Oct;10(5):501-506. 10.4162/nrp.2016.10.5.501.
Corn silk extract improves cholesterol metabolism in C57BL/6J mouse fed high-fat diets
- Affiliations
-
- 1Department of Food Science and Nutrition, Dankook University, 152, Juljeon-ro, Suji-gu, Yonin-si, Gyeonggi 16890, Korea. wkkim@dankook.ac.kr
- 2Agriculture Science Technology, 300 Nongsaengmyeong-ro, Wansan-gu, Jeonju-si, Jeonbuk 54875, Korea.
- 3Department of Food Engineering, Dankook University, 119, Dandae-ro, Dongnam-gu, Cheonan-si, Chungnam 31116, Korea.
- KMID: 2434084
- DOI: http://doi.org/10.4162/nrp.2016.10.5.501
Abstract
- BACKGROUND/OBJECTIVES
Corn silk (CS) extract contains large amounts of maysin, which is a major flavonoid in CS. However, studies regarding the effect of CS extract on cholesterol metabolism is limited. Therefore, the purpose of this study was to determine the effect of CS extract on cholesterol metabolism in C57BL/6J mouse fed high-fat diets.
MATERIALS/METHODS
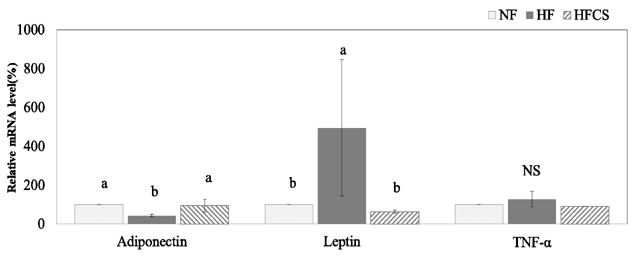
Normal-fat group fed 7% fat diet, high-fat (HF) group fed 25% fat diet, and high-fat with corn silk (HFCS) group were orally administered CS extract (100 mg/kg body weight) daily. Serum and hepatic levels of total lipids, triglycerides, and total cholesterol as well as serum free fatty acid, glucose, and insulin levels were determined. The mRNA expression levels of acyl-CoA: cholesterol acyltransferase (ACAT), cholesterol 7-alpha hydroxylase (CYP7A1), farnesoid X receptor (FXR), lecithin cholesterol acyltransferase (LCAT), low-density lipoprotein receptor, 3-hyroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), adiponectin, leptin, and tumor necrosis factor α were determined.
RESULTS
Oral administration of CS extract with HF improved serum glucose and insulin levels as well as attenuated HF-induced fatty liver. CS extracts significantly elevated mRNA expression levels of adipocytokines and reduced mRNA expression levels of HMG-CoA reductase, ACAT, and FXR. The mRNA expression levels of CYP7A1 and LCAT between the HF group and HFCS group were not statistically different.
CONCLUSIONS
CS extract supplementation with a high-fat diet improves levels of adipocytokine secretion and glucose homeostasis. CS extract is also effective in decreasing the regulatory pool of hepatic cholesterol, in line with decreased blood and hepatic levels of cholesterol though modulation of mRNA expression levels of HMG-CoA reductase, ACAT, and FXR.
Keyword
MeSH Terms
-
Adipokines
Adiponectin
Administration, Oral
Animals
Blood Glucose
Cholesterol*
Diet
Diet, High-Fat*
Fatty Liver
Glucose
Homeostasis
Insulin
Leptin
Metabolism*
Mice*
Oxidoreductases
Phosphatidylcholine-Sterol O-Acyltransferase
Receptors, Lipoprotein
RNA, Messenger
Silk*
Sterol O-Acyltransferase
Triglycerides
Tumor Necrosis Factor-alpha
Zea mays*
Adipokines
Adiponectin
Cholesterol
Glucose
Insulin
Leptin
Oxidoreductases
Phosphatidylcholine-Sterol O-Acyltransferase
RNA, Messenger
Receptors, Lipoprotein
Silk
Sterol O-Acyltransferase
Triglycerides
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Corn silk extract improves benign prostatic hyperplasia in experimental rat model
So Ra Kim, Ae Wha Ha, Hyun Ji Choi, Sun Lim Kim, Hyeon Jung Kang, Myung Hwan Kim, Woo Kyoung Kim
Nutr Res Pract. 2017;11(5):373-380. doi: 10.4162/nrp.2017.11.5.373.
Reference
-
1. Koska J, Stefan N, Permana PA, Weyer C, Sonoda M, Bogardus C, Smith SR, Joanisse DR, Funahashi T, Krakoff J, Bunt JC. Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am J Clin Nutr. 2008; 87:295–302.
Article2. Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett. 2006; 580:2917–2921.
Article3. Doan DD, Nguyen NH, Doan HK, Nguyen TL, Phan TS, van Dau N, Grabe M, Johansson R, Lindgren G, Stjernström NE. Studies on the individual and combined diuretic effects of four Vietnamese traditional herbal remedies (Zea mays, Imperata cylindrica, Plantago major and Orthosiphon stamineus). J Ethnopharmacol. 1992; 36:225–231.
Article4. Hasanudin K, Hashim P, Mustafa S. Corn silk (Stigma maydis) in healthcare: a phytochemical and pharmacological review. Molecules. 2012; 17:9697–9715.
Article5. Kan A, Orhan I, Coksari G, Sener B. In-vitro neuroprotective properties of the Maydis stigma extracts from four corn varieties. Int J Food Sci Nutr. 2012; 63:1–4.
Article6. Velazquez DV, Xavier HS, Batista JE, de Castro-Chaves C. Zea mays L. extracts modify glomerular function and potassium urinary excretion in conscious rats. Phytomedicine. 2005; 12:363–369.
Article7. Zhao W, Yin Y, Yu Z, Liu J, Chen F. Comparison of anti-diabetic effects of polysaccharides from corn silk on normal and hyperglycemia rats. Int J Biol Macromol. 2012; 50:1133–1137.
Article8. Farsi DA, Harris CS, Reid L, Bennett SA, Haddad PS, Martineau LC, Arnason JT. Inhibition of non-enzymatic glycation by silk extracts from a Mexican land race and modern inbred lines of maize (Zea mays). Phytother Res. 2008; 22:108–112.
Article9. Suzuki R, Okada Y, Okuyama T. The favorable effect of style of Zea mays L. on streptozotocin induced diabetic nephropathy. Biol Pharm Bull. 2005; 28:919–920.
Article10. Guo J, Liu T, Han L, Liu Y. The effects of corn silk on glycaemic metabolism. Nutr Metab (Lond). 2009; 6:47.
Article11. Maksimović Z, Dobrić S, Kovacević N, Milovanović Z. Diuretic activity of Maydis stigma extract in rats. Pharmazie. 2004; 59:967–971.12. Bai H, Hai C, Xi M, Liang X, Liu R. Protective effect of maize silks (Maydis stigma) ethanol extract on radiation-induced oxidative stress in mice. Plant Foods Hum Nutr. 2010; 65:271–276.
Article13. Choi DJ, Kim SL, Choi JW, Park YI. Neuroprotective effects of corn silk maysin via inhibition of H2O2-induced apoptotic cell death in SK-N-MC cells. Life Sci. 2014; 109:57–64.
Article14. Maksimović Z, Malencić D, Kovacević N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour Technol. 2005; 96:873–877.
Article15. Kim SL, Kim MJ, Lee YY, Jung GH, Son BY, Lee JS, Kwon YU, Park YI. Isolation and identification of flavonoids from corn silk. Korean J Crop Sci. 2014; 59:435–444.
Article16. Waiss AC Jr, Chan BG, Elliger CA, Wiseman BR, McMillian WW, Widstrom NW, Zuber MS, Keaster AJ. Maysin, a flavone glycoside from corn silks with antibiotic activity toward corn earworm. J Econ Entomol. 1979; 72:256–258.
Article17. Lee EA, Byrne PF, McMullen MD, Snook ME, Wiseman BR, Widstrom NW, Coe EH. Genetic mechanisms underlying apimaysin and maysin synthesis and corn earworm antibiosis in maize (Zea mays L.). Genetics. 1998; 149:1997–2006.
Article18. Jeong HW, Lee JW, Kim WS, Choe SS, Kim KH, Park HS, Shin HJ, Lee GY, Shin D, Lee H, Lee JH, Choi EB, Lee HK, Chung H, Park SB, Park KS, Kim HS, Ro S, Kim JB. A newly identified CG301269 improves lipid and glucose metabolism without body weight gain through activation of peroxisome proliferator-activated receptor alpha and gamma. Diabetes. 2011; 60:496–506.
Article19. Min OJ, Sharma BR, Park CM, Rhyu DY. Effect of myadis stigma water extract on adipogenesis and blood glucose in 3T3-L1 adipocytes and db/db mice. Korean J Pharmacogn. 2011; 42:201–208.20. Schreyer SA, Wilson DL, LeBoeuf RC. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis. 1998; 136:17–24.
Article21. Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997; 127:838S–841S.
Article22. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509.
Article23. Ha AW, Kim WK. The effect of fucoxanthin rich power on the lipid metabolism in rats with a high fat diet. Nutr Res Pract. 2013; 7:287–293.
Article24. Dai W, Wang K, Zheng X, Chen X, Zhang W, Zhang Y, Hou J, Liu L. High fat plus high cholesterol diet lead to hepatic steatosis in zebrafish larvae: a novel model for screening anti-hepatic steatosis drugs. Nutr Metab (Lond). 2015; 12:42.
Article25. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006; 116:1784–1792.
Article26. Li Y, Qin G, Liu J, Mao L, Zhang Z, Shang J. Adipose tissue regulates hepatic cholesterol metabolism via adiponectin. Life Sci. 2014; 118:27–33.
Article27. Liu Q, Yuan B, Lo KA, Patterson HC, Sun Y, Lodish HF. Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism. Proc Natl Acad Sci U S A. 2012; 109:14568–14573.
Article28. Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoEdeficient mice from atherosclerosis. J Biol Chem. 2003; 278:2461–2468.
Article29. DeBose-Boyd RA. Feedback regulation of cholesterol synthesis: sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res. 2008; 18:609–621.
Article30. Krause BR, Pape ME, Kieft K, Auerbach B, Bisgaier CL, Homan R, Newton RS. ACAT inhibition decreases LDL cholesterol in rabbits fed a cholesterol-free diet. Marked changes in LDL cholesterol without changes in LDL receptor mRNA abundance. Arterioscler Thromb. 1994; 14:598–604.
Article31. Davignon J, Montigny M, Dufour R. HMG-CoA reductase inhibitors: a look back and a look ahead. Can J Cardiol. 1992; 8:843–864.32. Burnett JR, Wilcox LJ, Telford DE, Kleinstiver SJ, Barrett PH, Newton RS, Huff MW. Inhibition of HMG-CoA reductase by atorvastatin decreases both VLDL and LDL apolipoprotein B production in miniature pigs. Arterioscler Thromb Vasc Biol. 1997; 17:2589–2600.
Article33. Burnett JR, Wilcox LJ, Telford DE, Kleinstiver SJ, Barrett PH, Newton RS, Huff MW. Inhibition of ACAT by avasimibe decreases both VLDL and LDL apolipoprotein B production in miniature pigs. J Lipid Res. 1999; 40:1317–1327.
Article34. Llaverías G, Laguna JC, Alegret M. Pharmacology of the ACAT inhibitor avasimibe (CI-1011). Cardiovasc Drug Rev. 2003; 21:33–50.35. Evans MJ, Mahaney PE, Borges-Marcucci L, Lai K, Wang S, Krueger JA, Gardell SJ, Huard C, Martinez R, Vlasuk GP, Harnish DC. A synthetic farnesoid X receptor (FXR) agonist promotes cholesterol lowering in models of dyslipidemia. Am J Physiol Gastrointest Liver Physiol. 2009; 296:G543–G552.
Article36. Mencarelli A, Fiorucci S. FXR an emerging therapeutic target for the treatment of atherosclerosis. J Cell Mol Med. 2010; 14:79–92.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Liquid Culture of Coriolus Versicolor on Lipid Metabolism and Enzyme Activities in Rats Fed High Fat Diet
- High maysin corn silk extract reduces body weight and fat deposition in C57BL/6J mice fed high-fat diets
- Effects of Liquid Culture of Agaricus blazei Murill on Lipid Metabolism and Enzyme Activities in Rats Fed High Fat Diet
- Effects of Soyoligosaccharide on Lipid Metabolism in Rats Fed the High Fat or Low Fat Diet
- Effects of an aqueous extract of purple sweet potato on nonalcoholic fatty liver in high fat/cholesterol-fed mice