Int J Stem Cells.
2018 Nov;11(2):235-241. 10.15283/ijsc18036.
Mbd3 Promotes Reprogramming of Primary Human Fibroblasts
- Affiliations
-
- 1Department of Haematology, University College London, Cancer Institute, London, UK. amit.nathwani@ucl.ac.uk
- 2Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free NHS Trust, London, UK.
- 3National Health Services Blood and Transplant, Oak House, Reeds Crescent, Watford, Hertfordshire, UK.
- 4Centre for Paediatrics, Barts and The London Medical School, Blizard Institute, Queen Mary University of London, London, UK.
- KMID: 2429556
- DOI: http://doi.org/10.15283/ijsc18036
Abstract
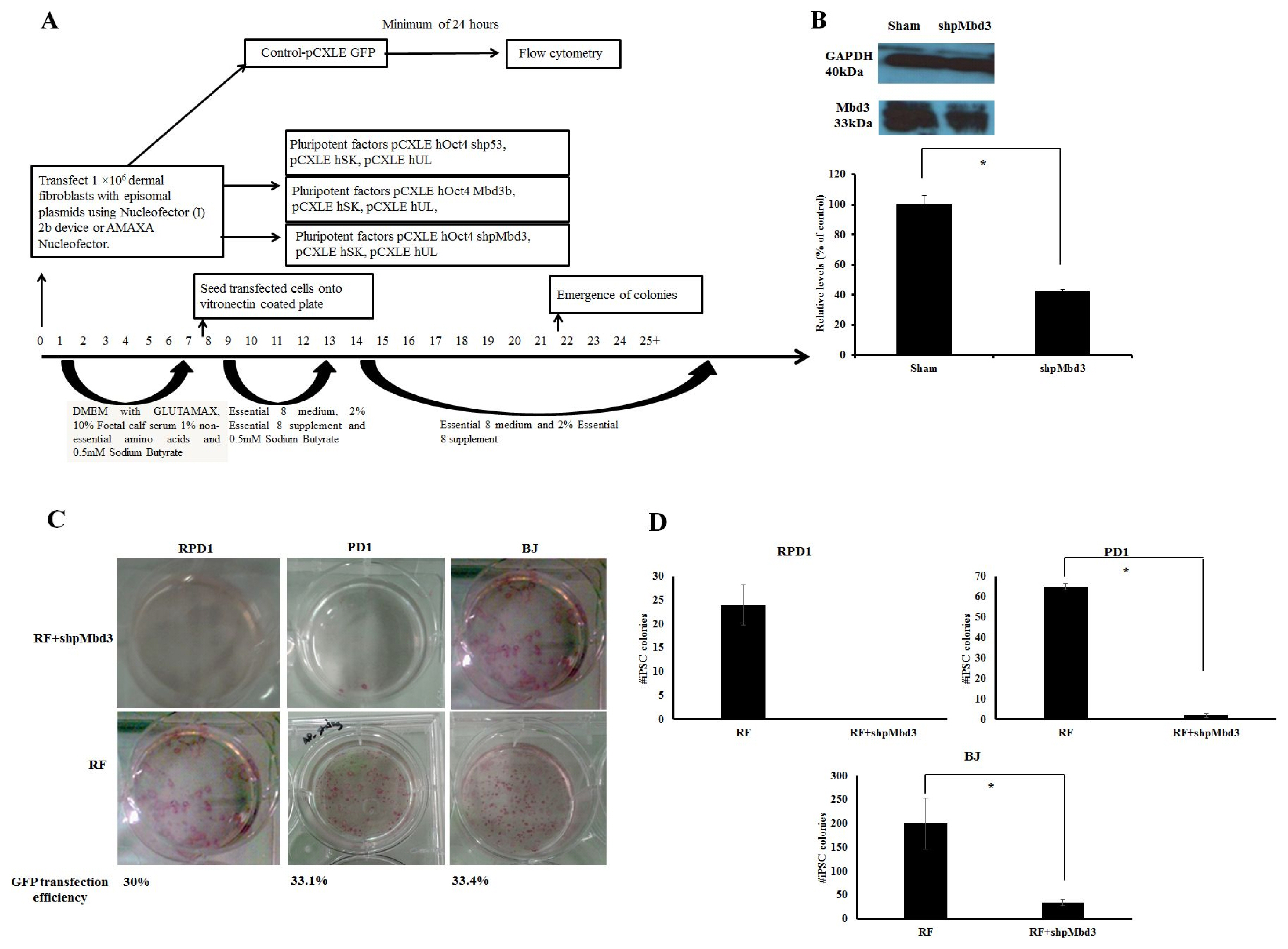
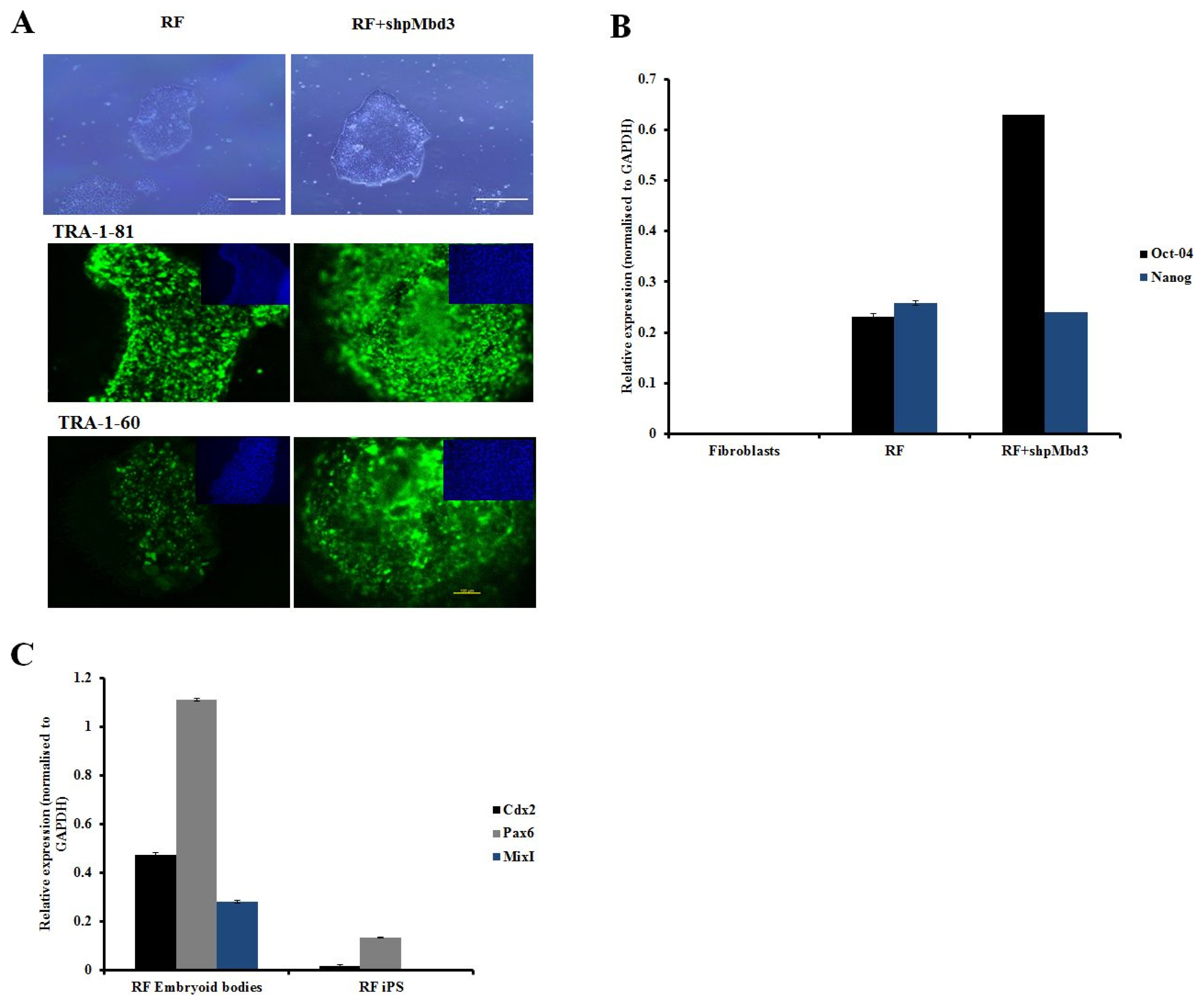
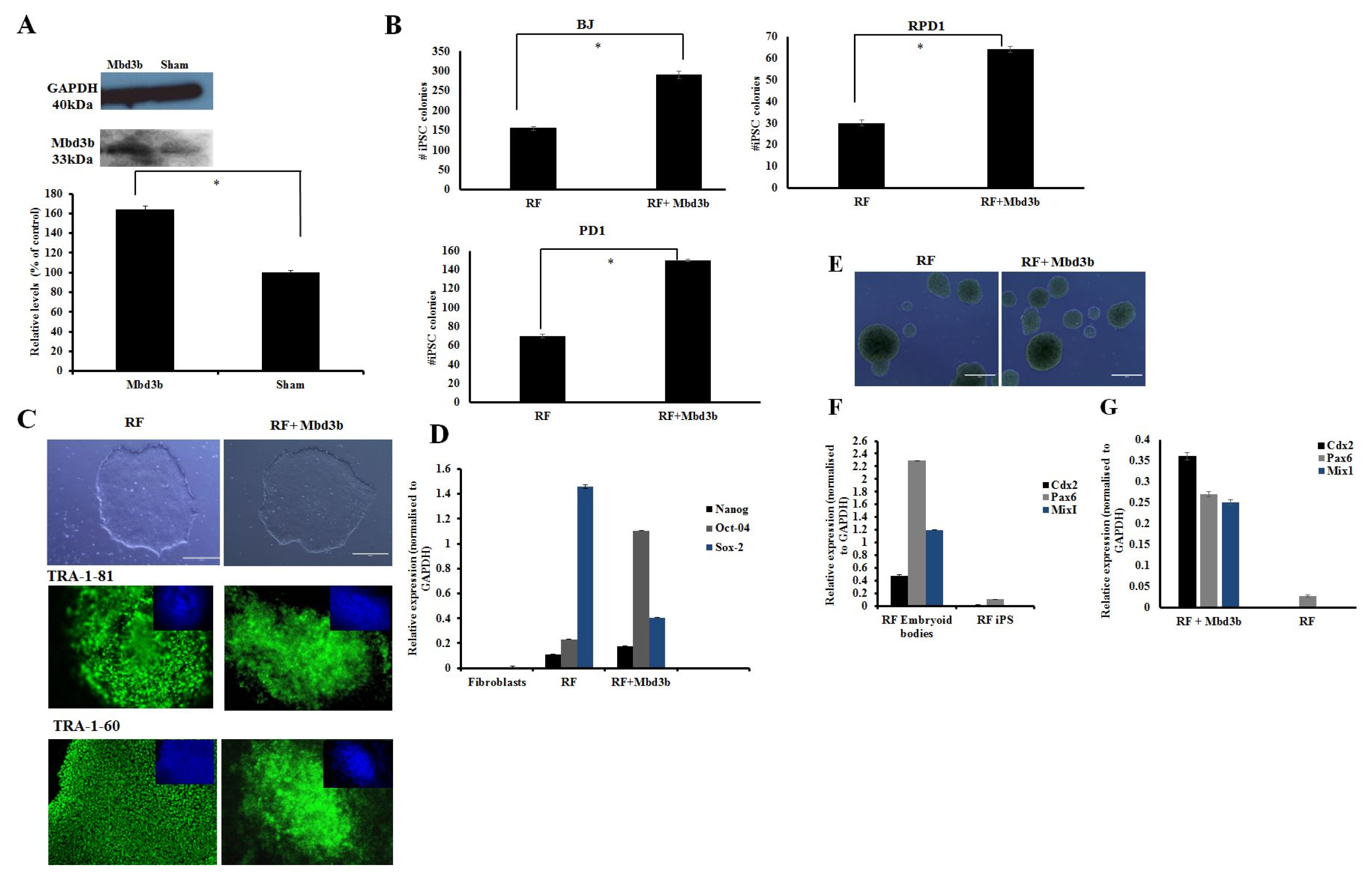
- Mbd3 (Methyl-CpG binding domain protein), a core member of NuRD (nucleosome remodelling and deacetylation) is essential for embryogenesis. However, its role in reprogramming of somatic cells into induced pluripotent stem cells (iPSC) remains controversial. Some reports suggest that Mbd3 inhibits pluripotency, whilst others show that it greatly enhances reprogramming efficiency. Our study is the first to assess the role of Mbd3 on reprogramming of primary human fibroblasts using Yamanaka episomal plasmids (Reprogramming factors (RF) under feeder-free conditions. We showed that shRNA-mediated partial depletion of Mbd3 resulted in >5-fold reduction in the efficiency of reprogramming of primary human fibroblasts. Furthermore, iPSC that emerged after knock-down of Mbd3 were incapable of trilineage differentiation even though they expressed all markers of pluripotency. In contrast, over-expression of the Mbd3b isoform along with the Yamanaka episomal plasmids increased the number of fibroblast derived iPSC colonies by at least two-fold. The resulting colonies were capable of trilineage differentiation. Our results, therefore, suggest that Mbd3 appears to play an important role in reprogramming of primary human fibroblasts, which provides further insight into the biology of reprogramming but also has direct implication for translation of iPSC to clinic.
MeSH Terms
Figure
Reference
-
References
1. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131:861–872. DOI: 10.1016/j.cell.2007.11.019. PMID: 18035408.
Article2. Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011; 8:409–412. DOI: 10.1038/nmeth.1591. PMID: 21460823.
Article3. Goh PA, Caxaria S, Casper C, Rosales C, Warner TT, Coffey PJ, Nathwani AC. A systematic evaluation of integration free reprogramming methods for deriving clinically relevant patient specific induced pluripotent stem (iPS) cells. PLoS One. 2013; 8:e81622. DOI: 10.1371/journal.pone.0081622. PMID: 24303062. PMCID: 3841145.
Article4. Kaji K, Nichols J, Hendrich B. Mbd3, a component of the NuRD co-repressor complex, is required for development of pluripotent cells. Development. 2007; 134:1123–1132. DOI: 10.1242/dev.02802. PMID: 17287250.
Article5. Hendrich B, Guy J, Ramsahoye B, Wilson VA, Bird A. Closely related proteins MBD2 and MBD3 play distinctive but interacting roles in mouse development. Genes Dev. 2001; 15:710–723. DOI: 10.1101/gad.194101. PMID: 11274056. PMCID: 312657.
Article6. Luo M, Ling T, Xie W, Sun H, Zhou Y, Zhu Q, Shen M, Zong L, Lyu G, Zhao Y, Ye T, Gu J, Tao W, Lu Z, Grummt I. NuRD blocks reprogramming of mouse somatic cells into pluripotent stem cells. Stem Cells. 2013; 31:1278–1286. DOI: 10.1002/stem.1374. PMID: 23533168.
Article7. Rais Y, Zviran A, Geula S, Gafni O, Chomsky E, Viukov S, Mansour AA, Caspi I, Krupalnik V, Zerbib M, Maza I, Mor N, Baran D, Weinberger L, Jaitin DA, Lara-Astiaso D, Blecher-Gonen R, Shipony Z, Mukamel Z, Hagai T, Gilad S, Amann-Zalcenstein D, Tanay A, Amit I, Novershtern N, Hanna JH. Deterministic direct reprogramming of somatic cells to pluripotency. Nature. 2013; 502:65–70. DOI: 10.1038/nature12587. PMID: 24048479.
Article8. dos Santos RL, Tosti L, Radzisheuskaya A, Caballero IM, Kaji K, Hendrich B, Silva JC. MBD3/NuRD facilitates induction of pluripotency in a context-dependent manner. Cell Stem Cell. 2014; 15:102–110. DOI: 10.1016/j.stem.2014.04.019. PMID: 24835571. PMCID: 4082719.
Article9. Onder TT, Kara N, Cherry A, Sinha AU, Zhu N, Bernt KM, Cahan P, Marcarci BO, Unternaehrer J, Gupta PB, Lander ES, Armstrong SA, Daley GQ. Chromatin-modifying enzymes as modulators of reprogramming. Nature. 2012; 483:598–602. DOI: 10.1038/nature10953. PMID: 22388813. PMCID: 3501145.
Article10. Roloff TC, Ropers HH, Nuber UA. Comparative study of methyl-CpG-binding domain proteins. BMC Genomics. 2003; 4:1. DOI: 10.1186/1471-2164-4-1. PMID: 12529184. PMCID: 149351.
Article11. Kaji K, Caballero IM, MacLeod R, Nichols J, Wilson VA, Hendrich B. The NuRD component Mbd3 is required for pluripotency of embryonic stem cells. Nat Cell Biol. 2006; 8:285–292. DOI: 10.1038/ncb1372. PMID: 16462733.
Article12. Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protoc. 2012; 7:2029–2040. DOI: 10.1038/nprot.2012.130. PMID: 23099485. PMCID: 3571618.
Article13. McIntosh J, Lenting PJ, Rosales C, Lee D, Rabbanian S, Raj D, Patel N, Tuddenham EG, Christophe OD, McVey JH, Waddington S, Nienhuis AW, Gray JT, Fagone P, Mingozzi F, Zhou SZ, High KA, Cancio M, Ng CY, Zhou J, Morton CL, Davidoff AM, Nathwani AC. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013; 121:3335–3344. DOI: 10.1182/blood-2012-10-462200. PMID: 23426947. PMCID: 3637010.
Article14. Štefková K, Procházkováv J, Pacherník J. Alkaline phosphatase in stem cells. Stem cells Int. 2015; DOI: 10.1155/2015/628368.
Article15. Buta C, David R, Dressel R, Emgård M, Fuchs C, Gross U, Healy L, Hescheler J, Kolar R, Martin U, Mikkers H, Müller FJ, Schneider RK, Seiler AE, Spielmann H, Weitzer G. Reconsidering pluripotency tests: do we still need teratoma assays? Stem Cell Res. 2013; 11:552–562. DOI: 10.1016/j.scr.2013.03.001. PMID: 23611953.
Article16. Zhang L, Zheng Y, Sun Y, Zhang Y, Yan J, Chen Z, Jiang H. MiR-134-Mbd3 axis regulates the induction of pluripotency. J Cell Mol Med. 2016; 20:1150–1158. DOI: 10.1111/jcmm.12805. PMID: 26929159. PMCID: 4882991.
Article17. Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, Nardone J, Laiho A, Tahiliani M, Sommer CA, Mostoslavsky G, Lahesmaa R, Orkin SH, Rodig SJ, Daley GQ, Rao A. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011; 8:200–213. DOI: 10.1016/j.stem.2011.01.008. PMID: 21295276. PMCID: 3134318.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reprogramming of Cancer Cells into Induced Pluripotent Stem Cells Questioned
- Comparison of Reprogramming Methods for Generation of Induced-Oligodendrocyte Precursor Cells
- Direct Reprogramming to Human Induced Neuronal Progenitors from Fibroblasts of Familial and Sporadic Parkinson’s Disease Patients
- Strategic Application of Epigenetic Regulators for Efficient Neuronal Reprogramming of Human Fibroblasts
- Energy Metabolism in Human Pluripotent Stem and Differentiated Cells Compared Using a Seahorse XF96 Extracellular Flux Analyzer