Yonsei Med J.
2018 Dec;59(10):1166-1173. 10.3349/ymj.2018.59.10.1166.
RPS15a Silencing Suppresses Cell Proliferation and Migration of Gastric Cancer
- Affiliations
-
- 1Department of Gastroenterology, Ningbo No. 2 Hospital, Ningbo, China. shidingyuhang@163.com
- 2Department of Gastroenterology, Henan University of Chinese Medicine, Zhengzhou, China. 1306348522@qq.com
- KMID: 2426330
- DOI: http://doi.org/10.3349/ymj.2018.59.10.1166
Abstract
- PURPOSE
Information on the possible role of the ribosomal protein S15a (RPS15a) in gastric cancer is scarce. The aim of this study was to evaluate the impact of RPS15a gene expression on the growth and cell cycle of gastric cancer cells in vitro and in vivo.
MATERIALS AND METHODS
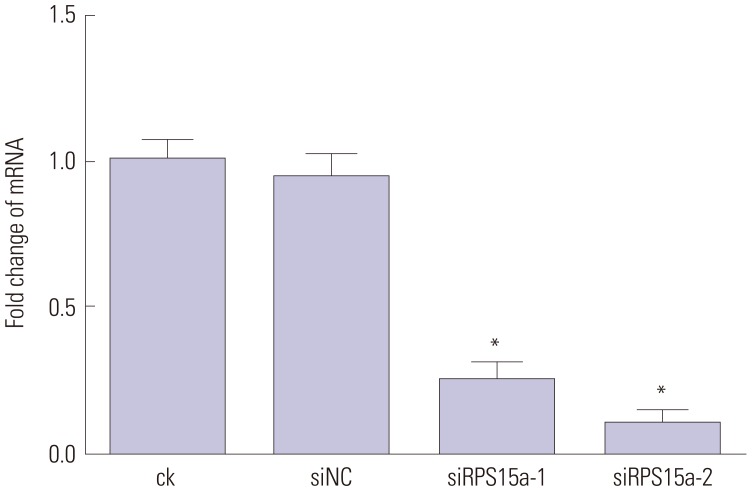
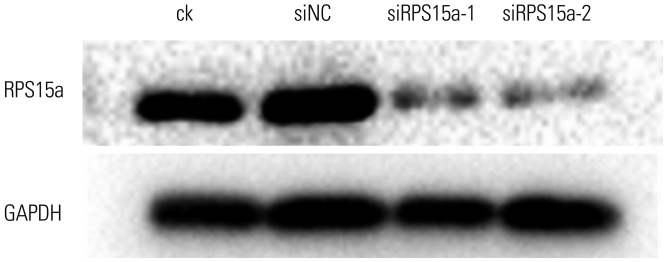
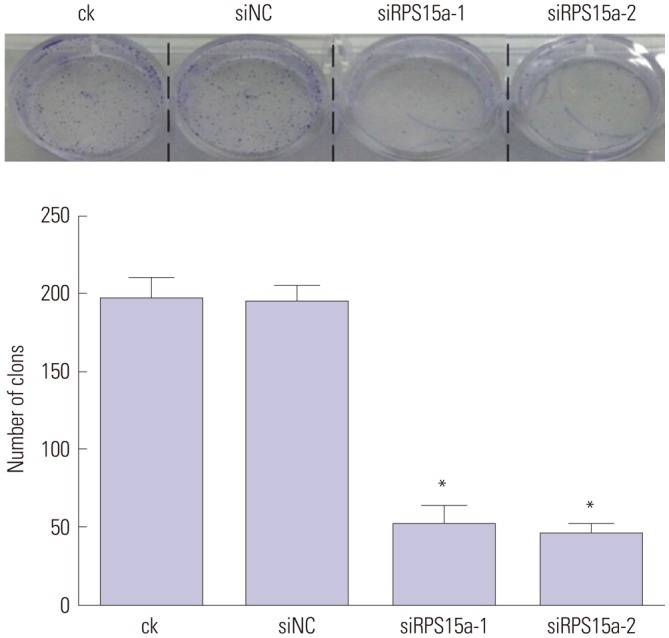
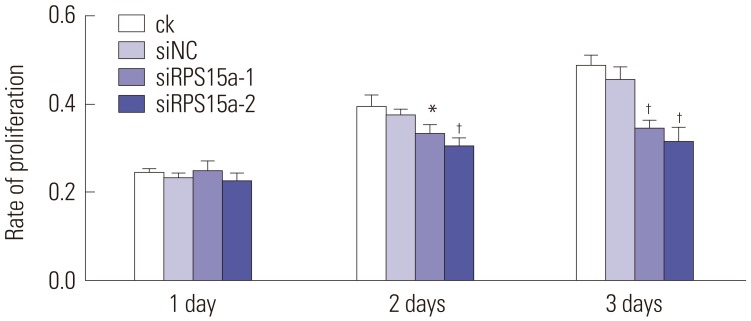
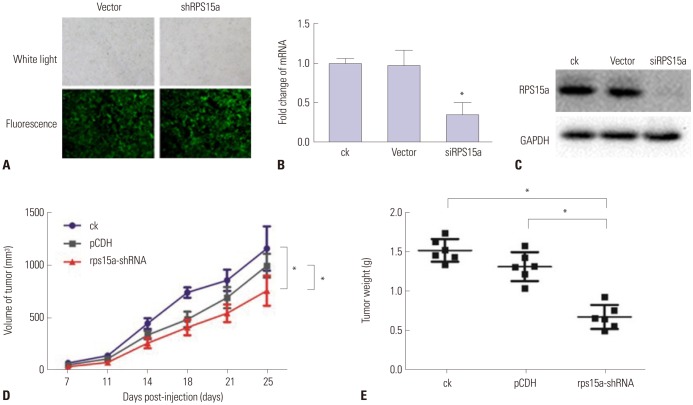
RPS15a mRNA expression was examined in cancer tissues and their corresponding adjacent normal tissues of 40 gastric adenocarcinoma patients. Next, RPS15a was knocked down using a lentivirus-mediated RNA interference (short hairpin RNA) system in the gastric cancer cell line BGC823. The effect of RPS15a knockdown was examined using CCK-8 assay, cell scratch test, colony formation assay, and flow cytometry. Finally, in nude mice, a tumorigenicity test was performed, and the tumor volume and weight were measured.
RESULTS
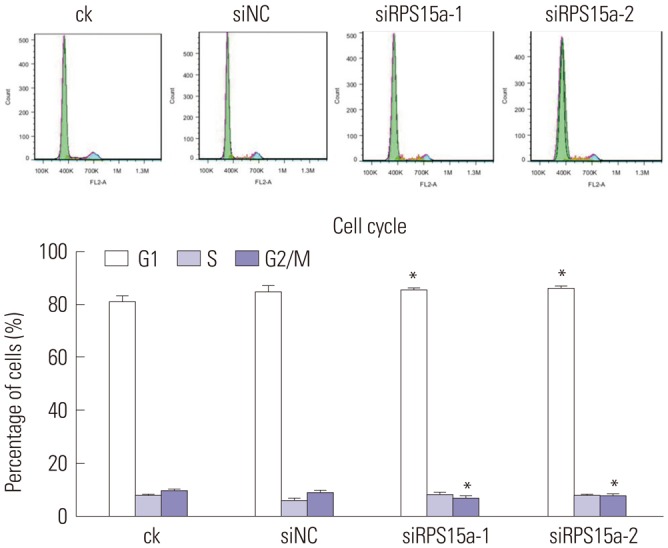
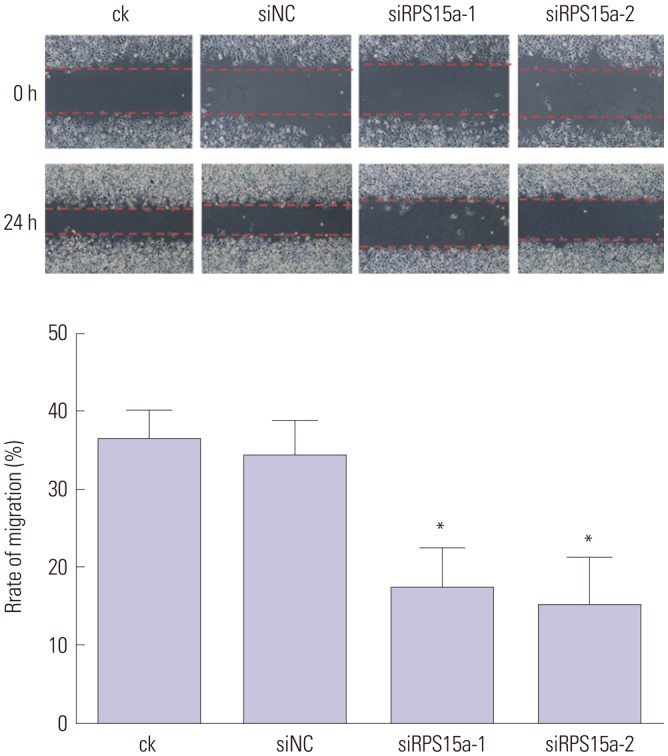
RPS15a expression in tumor tissue was significantly greater than that in the adjacent normal tissue of gastric cancer patients. After RPS15a silencing, the BGC823 cell proliferation rate decreased significantly; most cells were arrested in the G0/G1 phase, cell growth was inhibited, and the migration rate was decreased. Colony formation assay showed that the number and size of clones in the RPS15a-silenced cells were fewer and smaller, compared to control cells. The nude mouse tumorigenicity test showed that RPS15a silencing had an inhibitory effect on tumor volume and mice weight.
CONCLUSION
The present study found RPS15a expression to be higher in gastric tumors and its silencing in gastric cancer cells to inhibit the proliferation, growth, and migration thereof. Accordingly, RPS15a may be considered as a potential therapeutic target in gastric cancer.
Keyword
MeSH Terms
-
Adenocarcinoma
Animals
Carcinogenicity Tests
Cell Cycle
Cell Line
Cell Proliferation*
Clone Cells
Flow Cytometry
Gene Expression
Gene Silencing
Humans
In Vitro Techniques
Mice
Mice, Nude
Ribosomal Proteins
RNA Interference
RNA, Messenger
Sincalide
Stomach Neoplasms*
Tumor Burden
RNA, Messenger
Ribosomal Proteins
Sincalide
Figure
Reference
-
1. Zhang Y, Zhang G, Li X, Li B, Zhang X. The effect of ribosomal protein S15a in lung adenocarcinoma. PeerJ. 2016; 4:e1792. PMID: 26989627.
Article2. Li G, Zhang L, Liu J, Xiao T, Liu G, Wang J, et al. shRNA-mediated RPS15A silencing inhibits U937 acute myeloid leukemia cell proliferation and enhances apoptosis. Mol Med Rep. 2016; 13:4400–4406. PMID: 27035327.
Article3. Chen J, Wei Y, Feng Q, Ren L, He G, Chang W, et al. Ribosomal protein S15A promotes malignant transformation and predicts poor outcome in colorectal cancer through misregulation of p53 signaling pathway. Int J Oncol. 2016; 48:1628–1638. PMID: 26847263.
Article4. Xu M, Wang Y, Chen L, Pan B, Chen F, Fang Y, et al. Down-regulation of ribosomal protein S15A mRNA with a short hairpin RNA inhibits human hepatic cancer cell growth in vitro. Gene. 2014; 536:84–89. PMID: 24334120.
Article5. Zhang C, Zhang T, Song E, Himaya SW, Chen X, Zheng L. Ribosomal protein S15A augments human osteosarcoma cell proliferation in vitro. Cancer Biother Radiopharm. 2014; 29:451–456. PMID: 25409460.6. Lian Z, Liu J, Li L, Li X, Tufan NL, Wu MC, et al. Human S15a expression is upregulated by hepatitis B virus X protein. Mol Carcinog. 2004; 40:34–46. PMID: 15108328.
Article7. Zhao X, Shen L, Feng Y, Yu H, Wu X, Chang J, et al. Decreased expression of RPS15A suppresses proliferation of lung cancer cells. Tumour Biol. 2015; 36:6733–6740. PMID: 25833696.
Article8. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386. PMID: 25220842.
Article9. Akhondi-Meybodi M, Ghane M, Akhondi-Meybodi S, Dashti G. Five-year survival rate for gastric cancer in Yazd Province, Central Iran, from 2001 to 2008. Middle East J Dig Dis. 2017; 9:39–48. PMID: 28316765.
Article10. Qiao YF, Chen CG, Yue J, Ma MQ, Ma Z, Yu ZT. Prognostic significance of preoperative and postoperative CK19 and CEA mRNA levels in peripheral blood of patients with gastric cardia cancer. World J Gastroenterol. 2017; 23:1424–1433. PMID: 28293089.
Article11. Baniak N, Senger JL, Ahmed S, Kanthan SC, Kanthan R. Gastric biomarkers: a global review. World J Surg Oncol. 2016; 14:212. PMID: 27514667.
Article12. Lazăr DC, Tăban S, Cornianu M, Faur A, Goldis¸ A. New advances in targeted gastric cancer treatment. World J Gastroenterol. 2016; 22:6776–6799. PMID: 27570417.
Article13. Jomrich G, Schoppmann SF. Targeted therapy in gastric cancer. Eur Surg. 2016; 48:278–284. PMID: 27795701.
Article14. Zhang C, Fu J, Xue F, Ryu B, Zhang T, Zhang S, et al. Knockdown of ribosomal protein S15A induces human glioblastoma cell apoptosis. World J Surg Oncol. 2016; 14:129. PMID: 27130037.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interaction between ACOT7 and LncRNA NMRAL2P via Methylation Regulates Gastric Cancer Progression
- Enhancement of Cell Migration by Corticotropin-Releasing Hormone (CRH) in Human Gastric Cancer Cell Line, MKN-28
- Circular RNA hsa_circ_0005556 Accelerates Gastric Cancer Progression by Sponging miR-4270 to Increase MMP19 Expression
- Kruppel-like factor 4 mediates lysophosphatidic acid-stimulated migration and proliferation of PC3M prostate cancer cells
- Roles of Dopamine in Proliferation of Gastric-Cancer Cells