J Korean Ophthalmol Soc.
2018 Oct;59(10):946-952. 10.3341/jkos.2018.59.10.946.
Regulation of Matrix Metalloproteinase 2 Expression by an Adenosine A1 Agonist in Trabecular Meshwork Cells
- Affiliations
-
- 1Department of Ophthalmology, Daegu Catholic University School of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2422583
- DOI: http://doi.org/10.3341/jkos.2018.59.10.946
Abstract
- PURPOSE
We investigated the extent of adenosine A1 agonist-induced expression and regulation of matrix metalloproteinase 2 (MMP-2) synthesis in human trabecular meshwork cells (HTMC).
METHODS
Primary HTMC cultures were exposed to 0.1 or 1.0 µM N6-cyclohexyladenosine (CHA) for 2 h in the presence or absence of an inhibitor thereof, 8-cyclopentyl-1,3-dimethylxanthine (CPT). The expression level of mRNA encoding MMP-2 was assessed via reverse transcription-polymerase chain reaction, and the levels of tissue inhibitor of metalloproteinase 2 (TIMP2) and membrane-type-1 MMP (MT1-MMP) measured by Western blotting. The permeability of the HTMC monolayer was assessed with the aid of carboxyfluorescein.
RESULTS
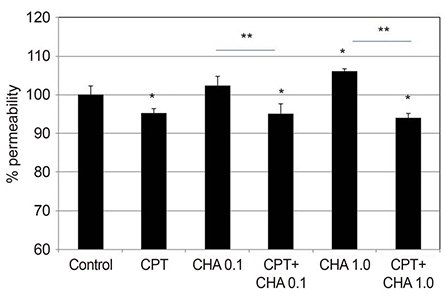
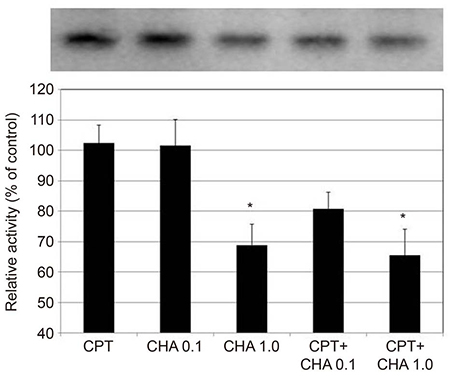
CHA at 1.0 µM increased the permeability of the HTMC monolayer (p = 0.003) and CHA at both 0.1 and 1.0 µM significantly increased MMP-2 mRNA expression, which was inhibited by co-exposure to CPT (all p < 0.05). CHA increased MMP-2 activity, decreased that of TIMP2, and increased that of MT1-MMP (all p < 0.05).
CONCLUSIONS
CHA increased the permeability of the HTMC monolayer and increased MMP-2 activity, decreased TIMP2 activity, and increased MT1-MMP activity. Thus, regulation of TIMP2 and MT1-MMP expression may be involved in the adenosine A1 agonist-induced increase in MMP-2 activity.
MeSH Terms
Figure
Cited by 1 articles
-
Effect and Mechanism of Phosphodiesterase Inhibitors on Trabecular Outflow
Jae Woo Kim, Jong Been Lee, So Hyung Lee
Korean J Ophthalmol. 2019;33(5):414-421. doi: 10.3341/kjo.2019.0057.
Reference
-
1. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–579.
Article2. Rohen JW, Lütjen-drecoll E, Flügel C, et al. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG). Exp Eye Res. 1993; 56:683–692.3. Schmidl D, Schmetterer D, Garhöfer G, Popa-Cherecheanu A. Pharmacotherapy of glaucoma. J Ocul Pharmacol Ther. 2015; 31:63–77.
Article4. Kopczynski CC, Epstein DL. Emerging trabecular outflow drugs. J Ocul Pharmacol Ther. 2014; 30:85–87.
Article5. Zhong Y, Yang Z, Huang WC, Luo X. Adenosine, adenosine receptors and glaucoma: an updated overview. Biochimica et Biophysica Acta. 2013; 1830:2882–2890.
Article6. Sanka K, Maddala R, Epstein DL, Rao PV. Influence of actin cytoskeletal integrity on matrix metalloproteinase-2 activation in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007; 48:2105–2114.
Article7. Shearer TW, Crosson CE. Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002; 43:3016–3020.8. Kim JW. Comparative study of the effects of trabecular meshwork outflow drugs on the permeability and nitric oxide production in trabecular meshwork cells. Korean J Ophthalmol. 2017; 31:452–459.
Article9. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article10. Song J, Deng PF, Stinnett SS, et al. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci. 2005; 46:2424–2432.
Article11. Kameda T, Inoue T, Inatani M, et al. The effect of Rho-associated protein kinase inhibitor on monkey Schlemm’s canal endothelial cells. Invest Ophthalmol Vis Sci. 2012; 53:3092–3103.
Article12. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–1049.13. Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–478.14. Crosson CE. Adenosine receptor activation modulates intraocular pressure in rabbits. J Pharmacol Exp Ther. 1995; 273:320–326.15. Crosson CE. Intraocular pressure responses to the adenosine agonist cyclohexyladenosine: evidence for a dual mechanism of action. Invest Ophthalmol Vis Sci. 2001; 42:1837–1840.16. Tian B, Gabelt BT, Crosson CE, Kaufman PL. Effects of adenosine agonists on intraocular pressure and aqueous humor dynamics in cynomolgus monkeys. Exp Eye Res. 1997; 64:979–989.
Article17. Husain S, Shearer TW, Crosson CE. Mechanisms linking adenosine A1 receptors and extracellular signal-regulated kinase 1/2 activation in human trabecular meshwork cells. J Pharmacol Exp Ther. 2007; 320:258–265.
Article18. Fleischhauer JC, Mitchell CH, Stamer WD, et al. Common actions of adenosine receptor agonists in modulating human trabecular meshwork cell transport. J Membrane Biol. 2003; 193:121–136.
Article19. Keller KE, Aga M, Bradley JM, et al. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009; 88:676–682.
Article20. Bradley JM, Vranka J, Colvis CM, et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998; 39:2649–2658.21. Pang IH, Helberg PE, Fleenor DL, et al. Expression of matrix metalloproteinases and their inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003; 44:3485–3493.
Article22. Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003; 59:812–823.
Article23. Löffek S, Schilling O, Franzke CW. Series “matrix metalloproteinases in lung health and disease”: biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011; 38:191–208.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Prostaglandin E2 Agonist Omidenepag on the Expression of Matrix Metalloproteinase in Trabecular Meshwork Cells
- Comparative Study of the Effects of Trabecular Meshwork Outflow Drugs on the Permeability and Nitric Oxide Production in Trabecular Meshwork Cells
- Effect of Nitric Oxide on the Expression of Matrix Metalloproteinase and Its Association with Migration of Cultured Trabecular Meshwork Cells
- Adenovirus Vector-mediated Gene Transfer into Human Trabecular Cell
- Regulation of the Levels of Trabecular Matrix Metalloproteinase and Inhibitor by Transforming Growth Factor-beta1