Allergy Asthma Immunol Res.
2018 Jul;10(4):406-419. 10.4168/aair.2018.10.4.406.
Tolerogenic Dendritic Cells Reduce Airway Inflammation in a Model of Dust Mite Triggered Allergic Inflammation
- Affiliations
-
- 1Instituto Gonçalo Moniz, Fundação Oswaldo Cruz (FIOCRUZ), Salvador, Bahia, Brazil. milena@bahia.fiocruz.br
- 2Faculdade de Medicina, Universidade Federal da Bahia, Salvador, Bahia, Brazil.
- 3Instituto de Ciências da Saúde, Universidade Federal da Bahia, Salvador, Bahia, Brazil.
- 4Hospital Universitário Edgard Santos, Universidade Federal da Bahia, Salvador, Bahia, Brazil.
- 5Centro de Biotecnologia e Terapia Celular, Hospital São Rafael, Salvador, Bahia, Brazil.
- 6Department of Diagnostics and Biomedical Sciences at The University of Texas Health Science Center, Houston, USA.
- 7Departamento de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil.
- KMID: 2421675
- DOI: http://doi.org/10.4168/aair.2018.10.4.406
Abstract
- PURPOSE
The use of tolerogenic dendritic cells (TolDCs) to control exacerbated immune responses may be a prophylactic and therapeutic option for application in autoimmune and allergic conditions. The objective of this work was to evaluate the effects of TolDC administration in a mouse model of allergic airway inflammation caused by mite extract.
METHODS
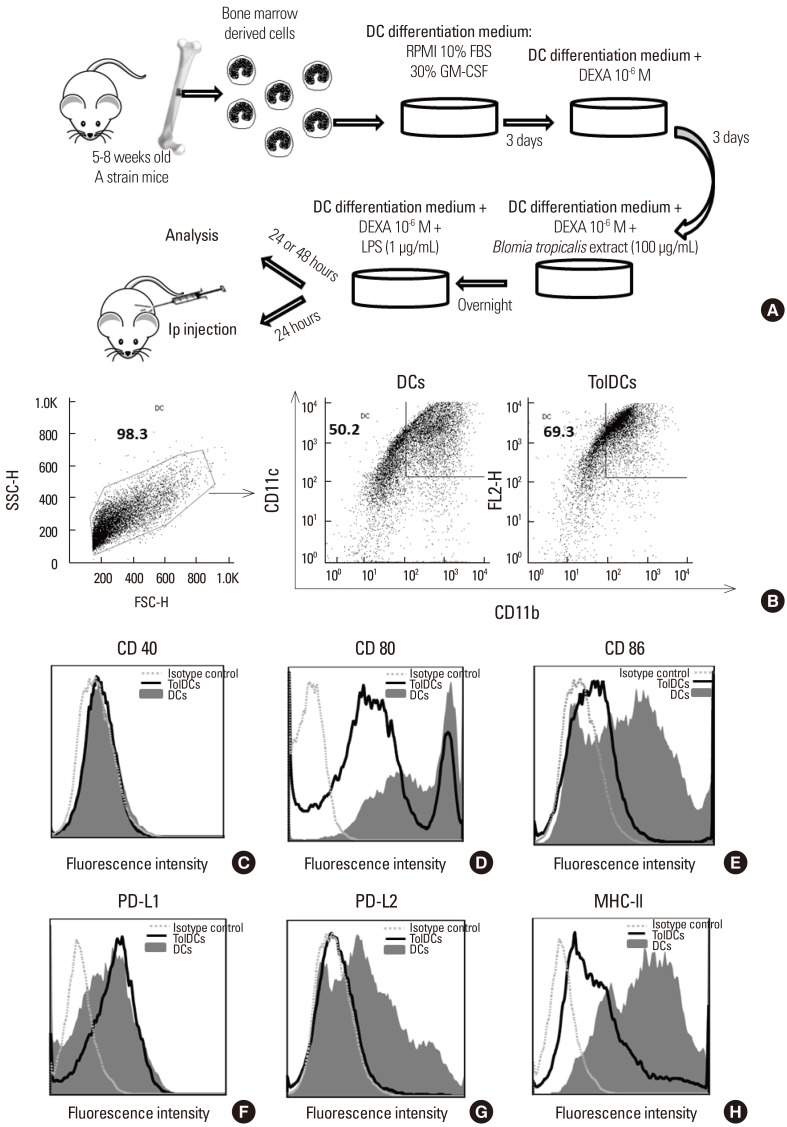
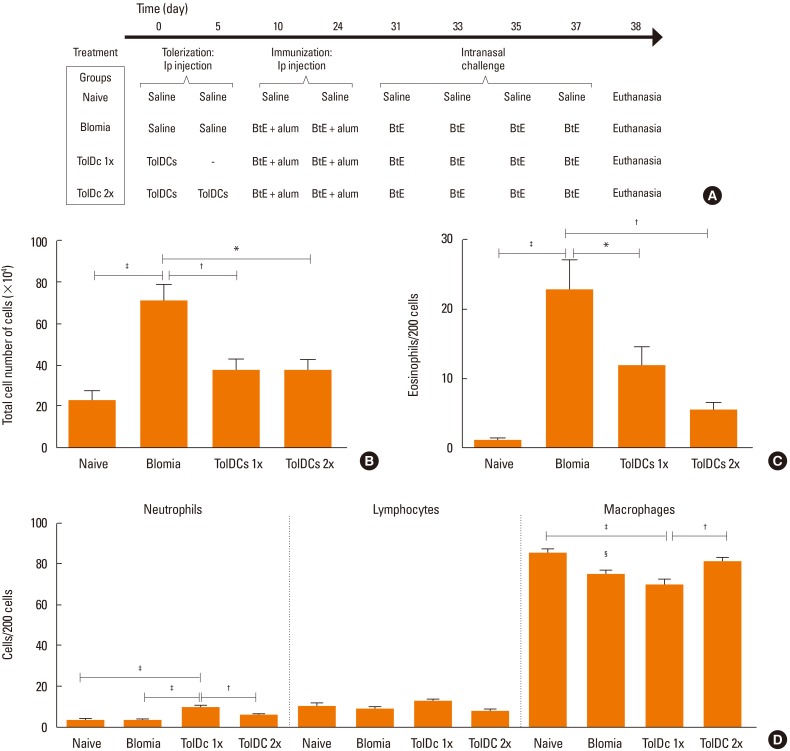
Mouse bone marrow-derived TolDCs were induced by incubation with granulocyte-macrophage colony-stimulating factor (GM-CSF) and dexamethasone, and then characterized by flow cytometry and cytokine production by enzyme-linked immunosorbent assay (ELISA). For the in vivo model of Blomia tropicalis-induced allergy, mice transplanted with antigen-pulsed TolDCs were sensitized intraperitoneally with B. tropicalis mite extract (BtE) adsorbed to aluminium hydroxide. After challenge by nasal administration of BtE, bronchoalveolar lavage fluid (BALF), lungs, spleen and serum were collected for analysis.
RESULTS
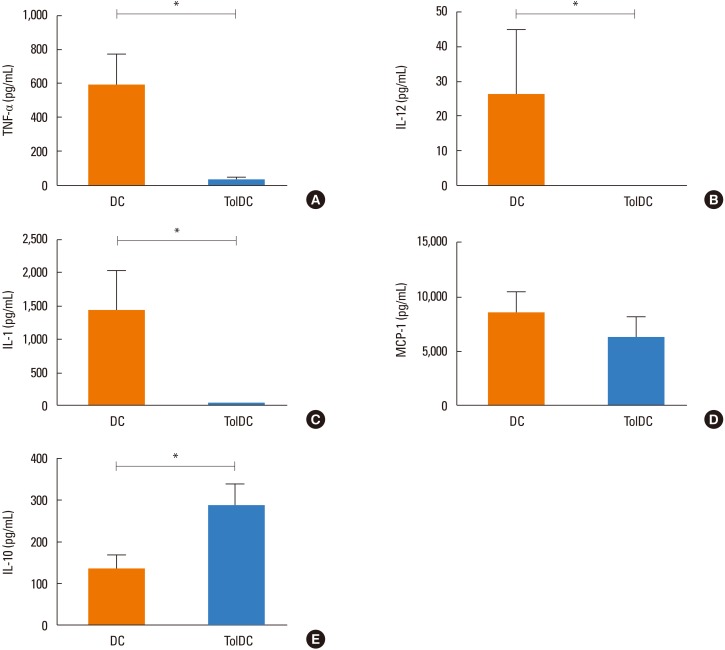
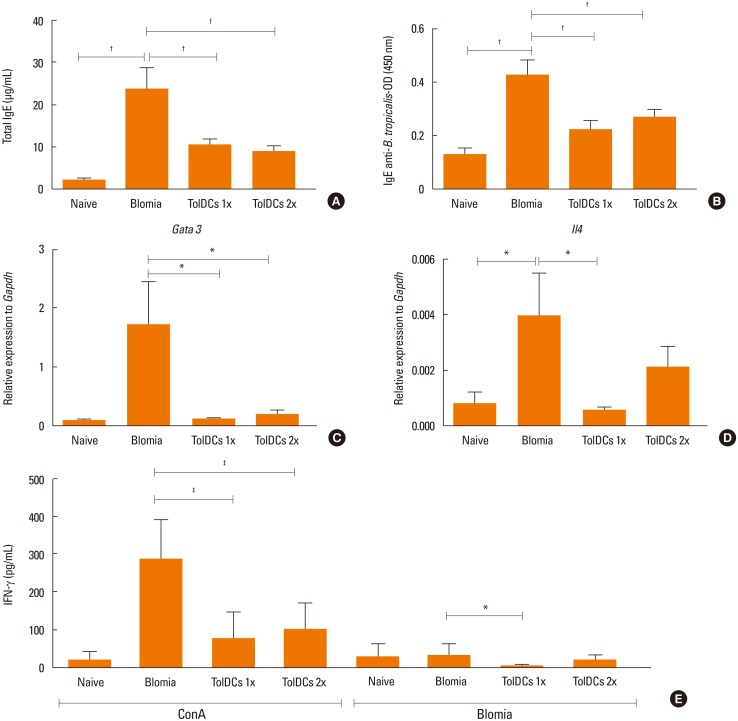
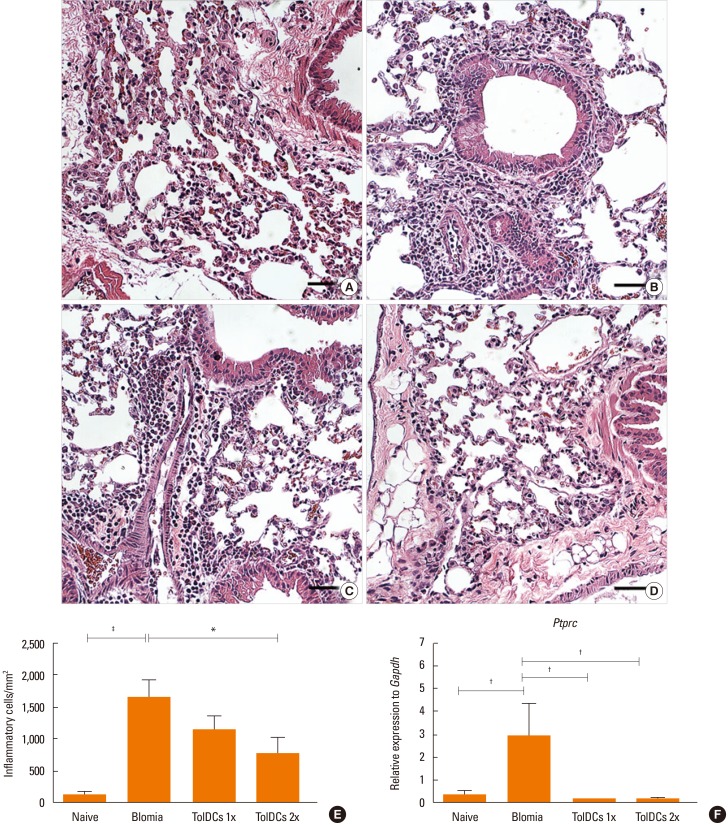
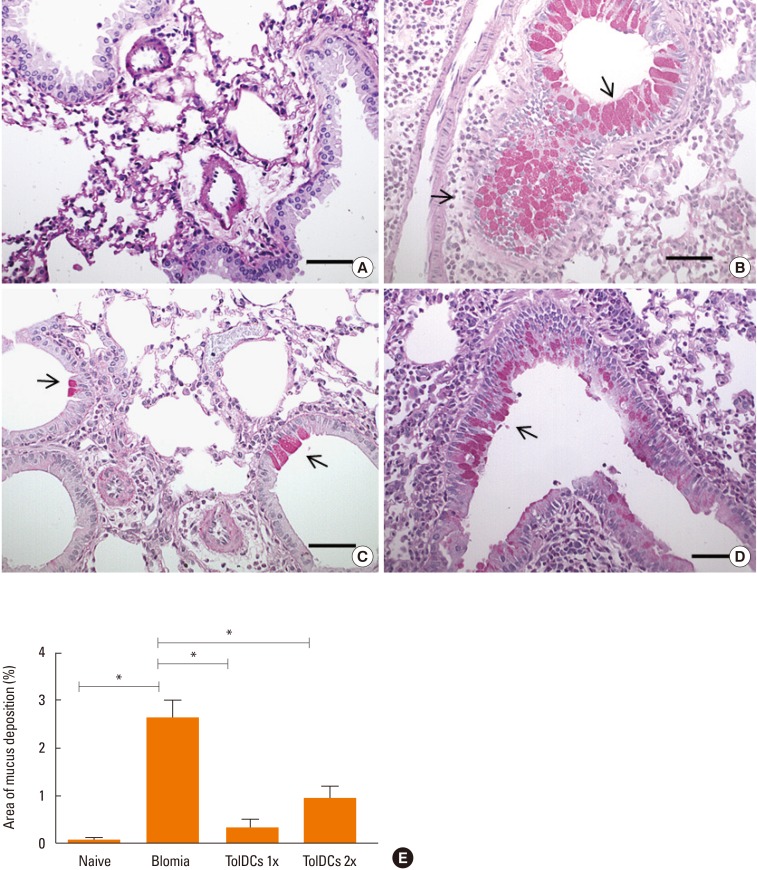
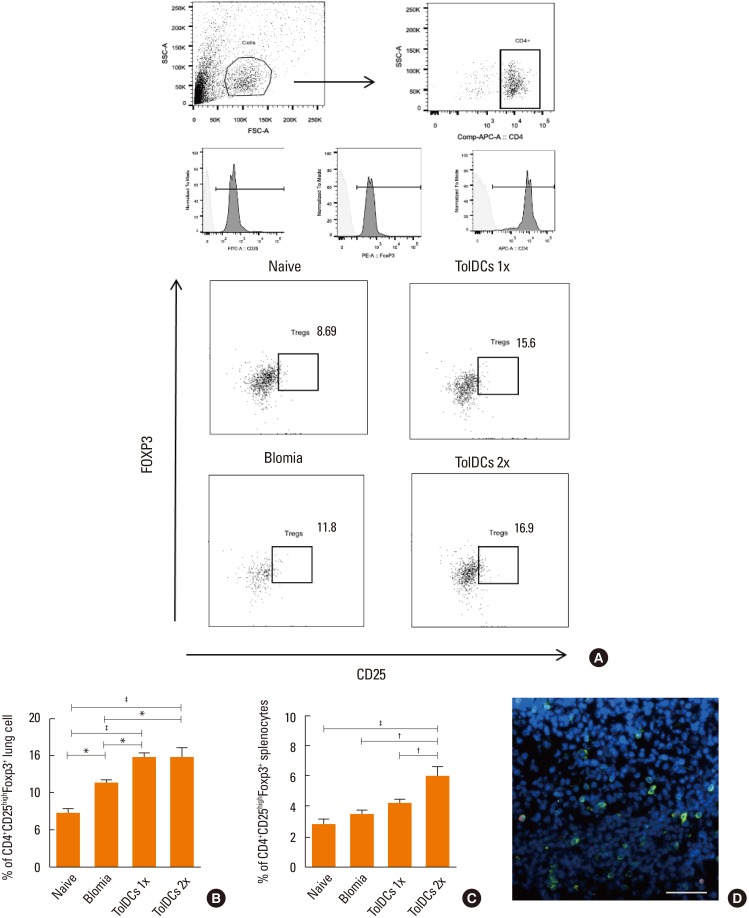
Induction of TolDCs was efficiently achieved as shown by low expression of major histocompatibility complex (MHC) II, programmed death-ligand (PD-L) 2 and pro-inflammatory cytokine production, and up-regulation of interleukin (IL)-10, upon LPS stimulation in vitro. Transplantation of 1 or 2 doses of BtE-pulsed TolDCs reduced the number of inflammatory cells in BALF and lungs as well as mucus deposition. Moreover, compared to saline-injected controls, TolDC-treated mice showed lower serum levels of anti-BtE immunoglobulin E (IgE) antibodies as well as reduced Gata3 and IL-4 gene expression in the lungs and decreased IFN-γ levels in the supernatant of splenocyte cultures Transplantation of TolDCs increased the percentage of the regulatory T cells in the spleen and the lungs.
CONCLUSIONS
Preventive treatment with TolDCs protects against dust mite-induced allergy in a mouse model, reinforcing the use of tolerogenic dendritic cells for the management of allergic conditions.
MeSH Terms
-
Administration, Intranasal
Animals
Antibodies
Antigens, Dermatophagoides
Asthma
Bronchoalveolar Lavage Fluid
Dendritic Cells*
Dexamethasone
Dust*
Enzyme-Linked Immunosorbent Assay
Flow Cytometry
Gene Expression
Granulocyte-Macrophage Colony-Stimulating Factor
Hypersensitivity
Immunoglobulin E
Immunoglobulins
In Vitro Techniques
Inflammation*
Interleukin-4
Interleukins
Lung
Major Histocompatibility Complex
Mice
Mites*
Mucus
Spleen
T-Lymphocytes, Regulatory
Up-Regulation
Antibodies
Antigens, Dermatophagoides
Dexamethasone
Dust
Granulocyte-Macrophage Colony-Stimulating Factor
Immunoglobulin E
Immunoglobulins
Interleukin-4
Interleukins
Figure
Cited by 1 articles
-
An Alternative Dendritic Cell-Induced Murine Model of Asthma Exhibiting a Robust Th2/Th17-Skewed Response
Sang Chul Park, Hongmin Kim, Yeeun Bak, Dahee Shim, Kee Woong Kwon, Chang-Hoon Kim, Joo-Heon Yoon, Sung Jae Shin
Allergy Asthma Immunol Res. 2020;12(3):537-555. doi: 10.4168/aair.2020.12.3.537.
Reference
-
1. Ferrando M, Bagnasco D, Varricchi G, Bernardi S, Bragantini A, Passalacqua G, et al. Personalized medicine in allergy. Allergy Asthma Immunol Res. 2017; 9:15–24. PMID: 27826958.
Article2. Comaru T, Pitrez PM, Friedrich FO, Silveira VD, Pinto LA. Free asthma medications reduces hospital admissions in Brazil (free asthma drugs reduces hospitalizations in Brazil). Respir Med. 2016; 121:21–25. PMID: 27888987.3. Mizutani N, Nabe T, Yoshino S. Interleukin-33 and alveolar macrophages contribute to the mechanisms underlying the exacerbation of IgE-mediated airway inflammation and remodelling in mice. Immunology. 2013; 139:205–218. PMID: 23323935.
Article4. Cabral AL, Sousa AW, Mendes FA, de Carvalho CR. Phenotypes of asthma in low-income children and adolescents: cluster analysis. J Bras Pneumol. 2017; 43:44–50. PMID: 28125150.
Article5. Baqueiro T, Carvalho FM, Rios CF, dos Santos NM, Alcântara-Neves NM. Dust mite species and allergen concentrations in beds of individuals belonging to different urban socioeconomic groups in Brazil. J Asthma. 2006; 43:101–105. PMID: 16517425.
Article6. Lee JH, Kim SC, Choi H, Jung CG, Ban GY, Shin YS, et al. A retrospective study of clinical response predictors in subcutaneous allergen immunotherapy with house dust mites for allergic rhinitis. Allergy Asthma Immunol Res. 2018; 10:18–24. PMID: 29178674.
Article7. Walker C, Zuany-Amorim C. New trends in immunotherapy to prevent atopic diseases. Trends Pharmacol Sci. 2001; 22:84–90. PMID: 11166852.
Article8. Kandeel M, Balaha M, Inagaki N, Kitade Y. Current and future asthma therapies. Drugs Today (Barc). 2013; 49:325–339. PMID: 23724412.
Article9. Page CP, Spina D. Beta2-agonists and bronchial hyperresponsiveness. Clin Rev Allergy Immunol. 2006; 31:143–162. PMID: 17085790.10. Kon OM, Sihra BS, Loh LC, Barkans J, Compton CH, Barnes NC, et al. The effects of an anti-CD4 monoclonal antibody, keliximab, on peripheral blood CD4+ T-cells in asthma. Eur Respir J. 2001; 18:45–52. PMID: 11510804.
Article11. Gervais FG, Sawyer N, Stocco R, Hamel M, Krawczyk C, Sillaots S, et al. Pharmacological characterization of MK-7246, a potent and selective CRTH2 (chemoattractant receptor-homologous molecule expressed on T-helper type 2 cells) antagonist. Mol Pharmacol. 2011; 79:69–76. PMID: 20943773.
Article12. Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012; 380:651–659. PMID: 22901886.
Article13. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012; 18:716–725. PMID: 22561835.
Article14. Maneechotesuwan K, Yao X, Ito K, Jazrawi E, Usmani OS, Adcock IM, et al. Suppression of GATA-3 nuclear import and phosphorylation: a novel mechanism of corticosteroid action in allergic disease. PLoS Med. 2009; 6:e1000076. PMID: 19436703.
Article15. Akdis CA. Allergy and hypersensitivity: mechanisms of allergic disease. Curr Opin Immunol. 2006; 18:718–726. PMID: 17029937.16. Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973; 137:1142–1162. PMID: 4573839.17. Kushwah R, Hu J. Dendritic cell apoptosis: regulation of tolerance versus immunity. J Immunol. 2010; 185:795–802. PMID: 20601611.
Article18. Ureta G, Osorio F, Morales J, Rosemblatt M, Bono MR, Fierro JA. Generation of dendritic cells with regulatory properties. Transplant Proc. 2007; 39:633–637. PMID: 17445563.
Article19. Li X, Yang A, Huang H, Zhang X, Town J, Davis B, et al. Induction of type 2 T helper cell allergen tolerance by IL-10-differentiated regulatory dendritic cells. Am J Respir Cell Mol Biol. 2010; 42:190–199. PMID: 19372244.
Article20. Zhang-Hoover J, Finn P, Stein-Streilein J. Modulation of ovalbumin-induced airway inflammation and hyperreactivity by tolerogenic APC. J Immunol. 2005; 175:7117–7124. PMID: 16301614.
Article21. Koya T, Matsuda H, Takeda K, Matsubara S, Miyahara N, Balhorn A, et al. IL-10-treated dendritic cells decrease airway hyperresponsiveness and airway inflammation in mice. J Allergy Clin Immunol. 2007; 119:1241–1250. PMID: 17353041.
Article22. Nayyar A, Dawicki W, Huang H, Lu M, Zhang X, Gordon JR. Induction of prolonged asthma tolerance by IL-10-differentiated dendritic cells: differential impact on airway hyperresponsiveness and the Th2 immunoinflammatory response. J Immunol. 2012; 189:72–79. PMID: 22634620.
Article23. Pedersen AE, Gad M, Kristensen NN, Haase C, Nielsen CH, Claesson MH. Tolerogenic dendritic cells pulsed with enterobacterial extract suppress development of colitis in the severe combined immunodeficiency transfer model. Immunology. 2007; 121:526–532. PMID: 17428312.
Article24. Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010; 207:2097–2111. PMID: 20819925.
Article25. Juliá-Serdá G, Cabrera-Navarro P, Acosta-Fernández O, Martín-Pérez P, García-Bello MA, Antó-Boqué J. Prevalence of sensitization to Blomia tropicalis among young adults in a temperate climate. J Asthma. 2012; 49:349–354. PMID: 22486531.26. Floderer M, Prchal-Murphy M, Vizzardelli C. Dendritic cell-secreted lipocalin2 induces CD8+ T-cell apoptosis, contributes to T-cell priming and leads to a TH1 phenotype. PLoS One. 2014; 9:e101881. PMID: 25010215.
Article27. Matyszak MK, Citterio S, Rescigno M, Ricciardi-Castagnoli P. Differential effects of corticosteroids during different stages of dendritic cell maturation. Eur J Immunol. 2000; 30:1233–1242. PMID: 10760813.
Article28. Zhou Q, Ho AW, Schlitzer A, Tang Y, Wong KH, Wong FH, et al. GM-CSF–licened CD11b + lung dendritic cells orchestrate Th2 immunity to Blomia tropicalis. J Immunol. 2014; 193:496–509. PMID: 24943219.29. van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005; 201:981–991. PMID: 15781587.30. Svajger U, Rozman P. Tolerogenic dendritic cells: molecular and cellular mechanisms in transplantation. J Leukoc Biol. 2014; 95:53–69. PMID: 24108704.
Article31. Hubo M, Trinschek B, Kryczanowsky F, Tuettenberg A, Steinbrink K, Jonuleit H. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front Immunol. 2013; 4:82. PMID: 23565116.
Article32. Hammer GE, Ma A. Molecular control of steady-state dendritic cell maturation and immune homeostasis. Annu Rev Immunol. 2013; 31:743–791. PMID: 23330953.
Article33. Lan YY, Wang Z, Raimondi G, Wu W, Colvin BL, de Creus A, et al. ‘Alternatively activated’ dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006; 177:5868–5877. PMID: 17056511.
Article34. Lee JH, Park CS, Jang S, Kim JW, Kim SH, Song S, et al. Tolerogenic dendritic cells are efficiently generated using minocycline and dexamethasone. Sci Rep. 2017; 7:15087. PMID: 29118423.
Article35. Kvistborg P, Boegha M, Pedersen AW, Claesson MH, Zocca MB. Fast generation of dendritic cells. Cell Immunol. 2009; 260:56–62. PMID: 19818956.
Article36. Matsumoto K, Fukuyama S, Eguchi-Tsuda M, Nakano T, Matsumoto T, Matsumura M, et al. B7-DC induced by IL-13 works as a feedback regulator in the effector phase of allergic asthma. Biochem Biophys Res Commun. 2008; 365:170–175. PMID: 17981145.
Article37. Akbari O, Stock P, Singh AK, Lombardi V, Lee WL, Freeman GJ, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010; 3:81–91. PMID: 19741598.
Article38. Matsumoto K, Inoue H, Nakano T, Tsuda M, Yoshiura Y, Fukuyama S, et al. B7-DC regulates asthmatic response by an IFN-gamma-dependent mechanism. J Immunol. 2004; 172:2530–2541. PMID: 14764726.39. Lewkowich IP, Lajoie S, Stoffers SL, Suzuki Y, Richgels PK, Dienger K, et al. PD-L2 modulates asthma severity by directly decreasing dendritic cell IL-12 production. Mucosal Immunol. 2013; 6:728–739. PMID: 23149662.
Article40. Vasconcelos JF, Teixeira MM, Barbosa-Filho JM, Agra MF, Nunes XP, Giulietti AM, et al. Effects of umbelliferone in a murine model of allergic airway inflammation. Eur J Pharmacol. 2009; 609:126–131. PMID: 19289114.
Article41. Vasconcelos JF, Teixeira MM, Barbosa-Filho JM, Lúcio AS, Almeida JR, de Queiroz LP, et al. The triterpenoid lupeol attenuates allergic airway inflammation in a murine model. Int Immunopharmacol. 2008; 8:1216–1221. PMID: 18602067.
Article42. Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006; 6:761–771. PMID: 16998509.
Article43. Nakajima H, Takatsu K. Role of cytokines in allergic airway inflammation. Int Arch Allergy Immunol. 2007; 142:265–273. PMID: 17124428.
Article44. Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012; 18:673–683. PMID: 22561831.
Article45. Holgate ST, Arshad HS, Roberts GC, Howarth PH, Thurner P, Davies DE. A new look at the pathogenesis of asthma. Clin Sci (Lond). 2009; 118:439–450. PMID: 20025610.
Article46. Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009; 39:3147–3159. PMID: 19688742.47. Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten Brinke A. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction--a comparative study of human clinical-applicable DC. Clin Immunol. 2012; 142:332–342. PMID: 22225835.48. Wojas-Krawczyk K, Krawczyk P, Buczkowski J, Walkowska A, Jankowska O, Czekajska-Chehab E, et al. Immunotherapy of lung adenocarcinoma patient with Peptide-pulsed dendritic cells: a case report. Arch Immunol Ther Exp (Warsz). 2012; 60:69–77. PMID: 22143160.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Innate Immune Response in House Dust Mite-Induced Allergic Inflammation
- Erratum: Tolerogenic Dendritic Cells Reduce Airway Inflammation in a Model of Dust Mite Triggered Allergic Inflammation
- Role of Natural Killer Cells in Airway Inflammation
- House dust mite allergy: environment evaluation and disease prevention
- Stem cell therapy in animal models of allergic airway diseases