Clin Exp Vaccine Res.
2016 Jul;5(2):148-158. 10.7774/cevr.2016.5.2.148.
Protective efficacy and immune responses by homologous prime-booster immunizations of a novel inactivated Salmonella Gallinarum vaccine candidate
- Affiliations
-
- 1College of Veterinary Medicine, Chonbuk National University, Iksan, Korea. johnhlee@jbnu.ac.kr
- KMID: 2421562
- DOI: http://doi.org/10.7774/cevr.2016.5.2.148
Abstract
- PURPOSE
Salmonella enterica serovar Gallinarum (SG) ghost vaccine candidate was recently constructed. In this study, we evaluated various prime-boost vaccination strategies using the candidate strain to optimize immunity and protection efficacy against fowl typhoid.
MATERIALS AND METHODS
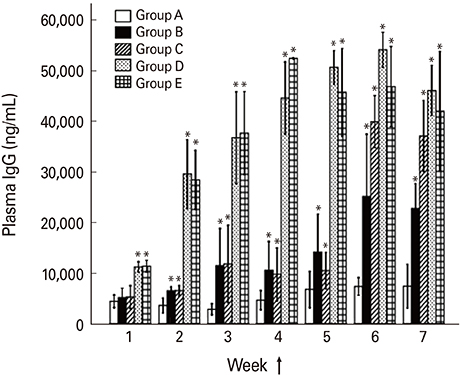
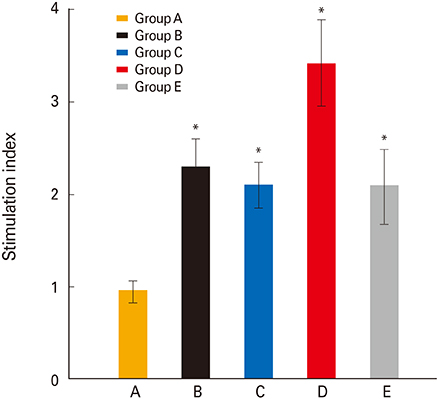
The chickens were divided into five groups designated as group A (non-immunized control), group B (orally primed and boosted), group C (primed orally and boosted intramuscularly), group D (primed and boosted intramuscularly), and group E (primed intramuscularly and boosted orally). The chickens were primed with the SG ghost at 7 days of age and were subsequently boosted at the fifth week of age. Post-immunization, the plasma IgG and intestinal secretory IgA (sIgA) levels, and the SG antigen-specific lymphocyte stimulation were monitored at weekly interval and the birds were subsequently challenged with a virulent SG strain at the third week post-second immunization.
RESULTS
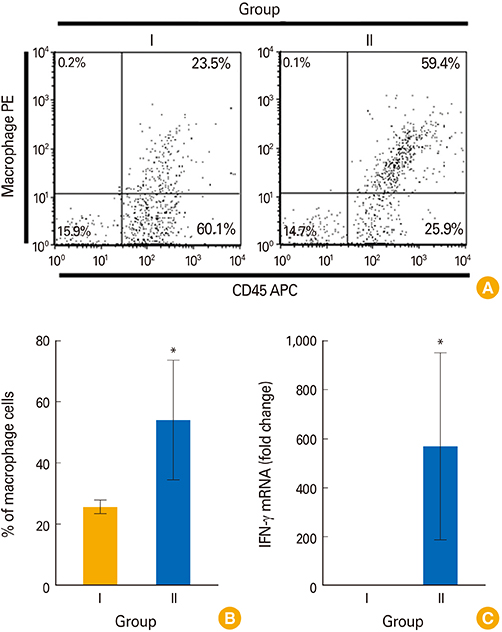
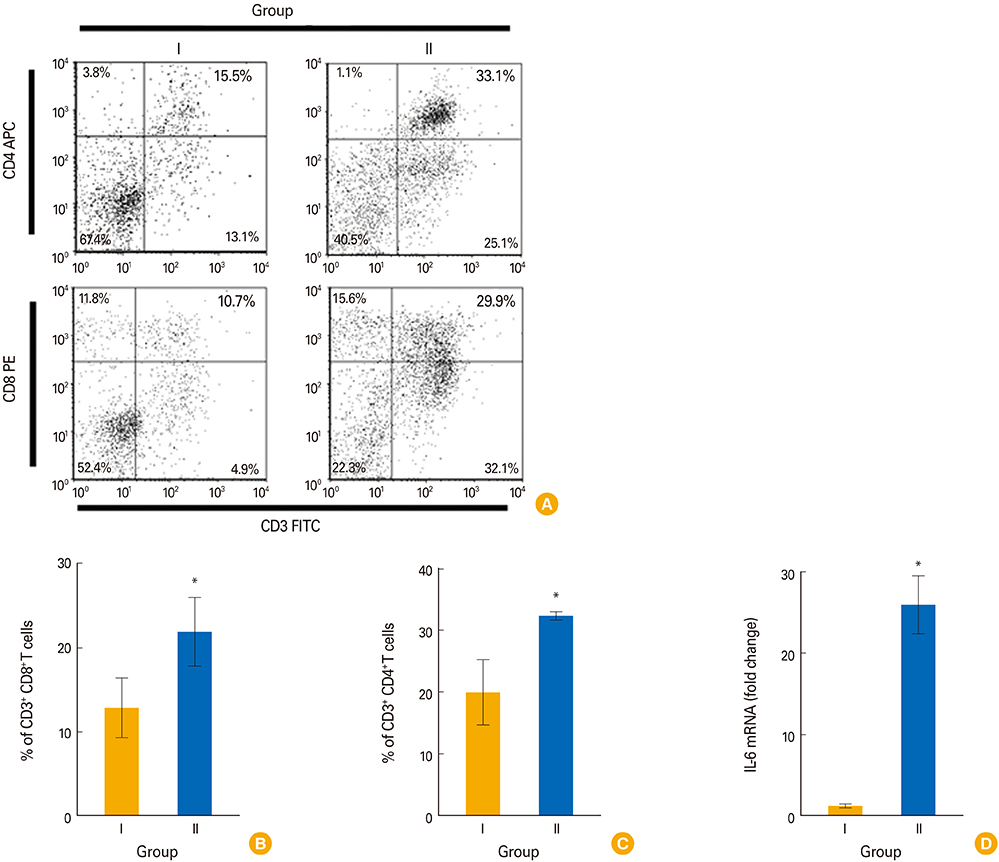
Chickens in group D showed an optimized protection with significantly increased plasma IgG, sIgA, and lymphocyte stimulation response compared to all groups. The presence of CD4+ and CD8+ T cells and monocyte/macrophage (M/M) in the spleen, and splenic expression of cytokines such as interferon γ (IFN-γ) and interleukin 6 (IL-6) in the immunized chickens were investigated. The prime immunization induced significantly higher splenic M/M population and mRNA levels of IFN-γ whereas the booster showed increases of splenic CD4+ and CD8+ T-cell population and IL-6 cytokine in mRNA levels.
CONCLUSION
Our results indicate that the prime immunization with the SG ghost vaccine induced Th1 type immune response and the booster elicited both Th1- and Th2-related immune responses.
Keyword
MeSH Terms
-
Birds
Chickens
Cytokines
Immunization*
Immunoglobulin A, Secretory
Immunoglobulin G
Interferons
Interleukin-6
Lymphocyte Activation
Plasma
RNA, Messenger
Salmonella enterica
Salmonella*
Serogroup
Spleen
T-Lymphocytes
Typhoid Fever
Vaccination
Cytokines
Immunoglobulin A, Secretory
Immunoglobulin G
Interferons
Interleukin-6
RNA, Messenger
Figure
Reference
-
1. Wray C, Wray A. Salmonella in domestic animals. Wallingford: CABI Publishing;2000.2. Pomeroy BS, Nagaraja KV. Fowl typhoid. Dis Poult. 1991; 9:87–99.3. Bouzoubaa K, Nagaraja KV, Kabbaj FZ, Newman JA, Pomeroy BS. Feasibility of using proteins from Salmonella gallinarum vs. 9R live vaccine for the prevention of fowl typhoid in chickens. Avian Dis. 1989; 33:385–391.
Article4. Chaudhari AA, Kim SW, Matsuda K, Lee JH. Safety evaluation and immunogenicity of arabinose-based conditional lethal Salmonella Gallinarum mutant unable to survive ex vivo as a vaccine candidate for protection against fowl typhoid. Avian Dis. 2011; 55:165–171.
Article5. Matsuda K, Chaudhari AA, Kim SW, Lee KM, Lee JH. Physiology, pathogenicity and immunogenicity of lon and/or cpxR deleted mutants of Salmonella Gallinarum as vaccine candidates for fowl typhoid. Vet Res. 2010; 41:59.
Article6. Matsuda K, Chaudhari AA, Lee JH. Evaluation of safety and protection efficacy on cpxR and lon deleted mutant of Salmonella Gallinarum as a live vaccine candidate for fowl typhoid. Vaccine. 2011; 29:668–674.
Article7. Rosu V, Chadfield MS, Santona A, et al. Effects of crp deletion in Salmonella enterica serotype Gallinarum. Acta Vet Scand. 2007; 49:14.
Article8. Shah DH, Shringi S, Desai AR, Heo EJ, Park JH, Chae JS. Effect of metC mutation on Salmonella Gallinarum virulence and invasiveness in 1-day-old White Leghorn chickens. Vet Microbiol. 2007; 119:352–357.
Article9. Smith HW. The use of live vaccines in experimental Salmonella gallinarum infection in chickens with observations on their interference effect. J Hyg (Lond). 1956; 54:419–432.
Article10. Zhang-Barber L, Turner AK, Dougan G, Barrow PA. Protection of chickens against experimental fowl typhoid using a nuoG mutant of Salmonella serotype Gallinarum. Vaccine. 1998; 16:899–903.
Article11. Kwon SR, Nam YK, Kim SK, Kim KH. Protection of tilapia (Oreochromis mosambicus) from edwardsiellosis by vaccination with Edwardsiella tarda ghosts. Fish Shellfish Immunol. 2006; 20:621–626.
Article12. Silva EN, Snoeyenbos GH, Weinack OM, Smyser CF. Studies on the use of 9R strain of Salmonella gallinarum as a vaccine in chickens. Avian Dis. 1981; 25:38–52.
Article13. Chaudhari AA, Jawale CV, Kim SW, Lee JH. Construction of a Salmonella Gallinarum ghost as a novel inactivated vaccine candidate and its protective efficacy against fowl typhoid in chickens. Vet Res. 2012; 43:44.
Article14. Jawale CV, Chaudhari AA, Lee JH. Generation of a safety enhanced Salmonella Gallinarum ghost using antibiotic resistance free plasmid and its potential as an effective inactivated vaccine candidate against fowl typhoid. Vaccine. 2014; 32:1093–1099.
Article15. Eko FO, Witte A, Huter V, et al. New strategies for combination vaccines based on the extended recombinant bacterial ghost system. Vaccine. 1999; 17:1643–1649.
Article16. Eko FO, Mayr UB, Attridge SR, Lubitz W. Characterization and immunogenicity of Vibrio cholerae ghosts expressing toxin-coregulated pili. J Biotechnol. 2000; 83:115–123.
Article17. Lubitz W, Witte A, Eko FO, et al. Extended recombinant bacterial ghost system. J Biotechnol. 1999; 73:261–273.
Article18. Ra CH, Kim YJ, Park SJ, et al. Evaluation of optimal culture conditions for recombinant ghost bacteria vaccine production with the antigen of Streptococcus iniae GAPDH. J Microbiol Biotechnol. 2009; 19:982–986.
Article19. Szostak MP, Hensel A, Eko FO, et al. Bacterial ghosts: non-living candidate vaccines. J Biotechnol. 1996; 44:161–170.
Article20. Jalava K, Hensel A, Szostak M, Resch S, Lubitz W. Bacterial ghosts as vaccine candidates for veterinary applications. J Control Release. 2002; 85:17–25.
Article21. Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002; 20:197–216.
Article22. Jechlinger W, Haller C, Resch S, Hofmann A, Szostak MP, Lubitz W. Comparative immunogenicity of the hepatitis B virus core 149 antigen displayed on the inner and outer membrane of bacterial ghosts. Vaccine. 2005; 23:3609–3617.
Article23. Mayr UB, Haller C, Haidinger W, et al. Bacterial ghosts as an oral vaccine: a single dose of Escherichia coli O157:H7 bacterial ghosts protects mice against lethal challenge. Infect Immun. 2005; 73:4810–4817.
Article24. Witte A, Wanner G, Blasi U, Halfmann G, Szostak M, Lubitz W. Endogenous transmembrane tunnel formation mediated by phi X174 lysis protein E. J Bacteriol. 1990; 172:4109–4114.
Article25. Witte A, Wanner G, Sulzner M, Lubitz W. Dynamics of PhiX174 protein E-mediated lysis of Escherichia coli. Arch Microbiol. 1992; 157:381–388.
Article26. McShane H. Prime-boost immunization strategies for infectious diseases. Curr Opin Mol Ther. 2002; 4:23–27.27. Hur J, Kim MY, Lee JH. Evaluation of efficacy of a new live Salmonella Typhimurium vaccine candidate in a murine model. Comp Immunol Microbiol Infect Dis. 2011; 34:171–177.
Article28. Kang HY, Srinivasan J, Curtiss R 3rd. Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar typhimurium vaccine. Infect Immun. 2002; 70:1739–1749.
Article29. Johnson J, Reid WM. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970; 28:30–36.
Article30. Song H, Yan R, Xu L, et al. Efficacy of DNA vaccines carrying Eimeria acervulina lactate dehydrogenase antigen gene against coccidiosis. Exp Parasitol. 2010; 126:224–231.
Article31. Nandre RM, Chaudhari AA, Matsuda K, Lee JH. Immunogenicity of a Salmonella Enteritidis mutant as vaccine candidate and its protective efficacy against salmonellosis in chickens. Vet Immunol Immunopathol. 2011; 144:299–311.
Article32. Porter RE Jr, Holt PS. Use of a pilocarpine-based lavage procedure to study secretory immunoglobulin concentration in the alimentary tract of White Leghorn chickens. Avian Dis. 1992; 36:529–536.
Article33. Gogal RM Jr, Ahmed SA, Larsen CT. Analysis of avian lymphocyte proliferation by a new, simple, nonradioactive assay (lympho-pro). Avian Dis. 1997; 41:714–725.
Article34. Meir R, Krispel S, Simanov L, Eliahu D, Maharat O, Pitcovski J. Immune responses to mucosal vaccination by the recombinant A1 and N proteins of infectious bronchitis virus. Viral Immunol. 2012; 25:55–62.
Article35. Park SI, Jeong JH, Choy HE, et al. Immune response induced by ppGpp-defective Salmonella enterica serovar Gallinarum in chickens. J Microbiol. 2010; 48:674–681.
Article36. Mittrucker HW, Kaufmann SH. Immune response to infection with Salmonella typhimurium in mice. J Leukoc Biol. 2000; 67:457–463.
Article37. Pirofski LA, Casadevall A. Use of licensed vaccines for active immunization of the immunocompromised host. Clin Microbiol Rev. 1998; 11:1–26.
Article38. Abd El, Jansen A, Clare S, et al. Candidate live, attenuated Salmonella enterica serotype Typhimurium vaccines with reduced fecal shedding are immunogenic and effective oral vaccines. Infect Immun. 2007; 75:1835–1842.
Article39. Kotton CN, Hohmann EL. Enteric pathogens as vaccine vectors for foreign antigen delivery. Infect Immun. 2004; 72:5535–5547.
Article40. Kwon HJ, Cho SH. Pathogenicity of SG 9R, a rough vaccine strain against fowl typhoid. Vaccine. 2011; 29:1311–1318.
Article41. Barrow PA, Huggins MB, Lovell MA. Host specificity of Salmonella infection in chickens and mice is expressed in vivo primarily at the level of the reticuloendothelial system. Infect Immun. 1994; 62:4602–4610.
Article42. Jeong JH, Song M, Park SI, Cho KO, Rhee JH, Choy HE. Salmonella enterica serovar gallinarum requires ppGpp for internalization and survival in animal cells. J Bacteriol. 2008; 190:6340–6350.
Article43. Peng W, Si W, Yin L, et al. Salmonella enteritidis ghost vaccine induces effective protection against lethal challenge in specific-pathogen-free chicks. Immunobiology. 2011; 216:558–565.
Article44. Kogut MH, McGruder ED, Hargis BM, Corrier DE, Deloach JR. Characterization of the pattern of inflammatory cell influx in chicks following the intraperitoneal administration of live Salmonella enteritidis and Salmonella enteritidis-immune lymphokines. Poult Sci. 1995; 74:8–17.
Article45. Roberts AD, Ordway DJ, Orme IM. Listeria monocytogenes infection in beta 2 microglobulin-deficient mice. Infect Immun. 1993; 61:1113–1116.
Article46. Farnell MB, El Halawani M, You S, McElroy AP, Hargis BM, Caldwell DJ. In vivo biologic effects of recombinant-turkey interferon-gamma in neonatal leghorn chicks: protection against Salmonella enteritidis organ invasion. Avian Dis. 2001; 45:473–478.
Article47. Okamura M, Lillehoj HS, Raybourne RB, Babu US, Heckert RA. Cell-mediated immune responses to a killed Salmonella enteritidis vaccine: lymphocyte proliferation, T-cell changes and interleukin-6 (IL-6), IL-1, IL-2, and IFN-gamma production. Comp Immunol Microbiol Infect Dis. 2004; 27:255–272.
Article48. Tellez GI, Kogut MH, Hargis BM. Immunoprophylaxis of Salmonella enteritidis infection by lymphokines in Leghorn chicks. Avian Dis. 1993; 37:1062–1070.
Article49. Chappell L, Kaiser P, Barrow P, Jones MA, Johnston C, Wigley P. The immunobiology of avian systemic salmonellosis. Vet Immunol Immunopathol. 2009; 128:53–59.
Article50. Lowenthal JW, Digby MR, York JJ. Production of interferon-gamma by chicken T cells. J Interferon Cytokine Res. 1995; 15:933–938.51. Kaspers B, Lillehoj HS, Jenkins MC, Pharr GT. Chicken interferon-mediated induction of major histocompatibility complex class II antigens on peripheral blood monocytes. Vet Immunol Immunopathol. 1994; 44:71–84.
Article52. Erf GF. Cell-mediated immunity in poultry. Poult Sci. 2004; 83:580–590.
Article53. Lalmanach AC, Lantier F. Host cytokine response and resistance to Salmonella infection. Microbes Infect. 1999; 1:719–726.
Article54. Schutt C. Fighting infection: the role of lipopolysaccharide binding proteins CD14 and LBP. Pathobiology. 1999; 67:227–229.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Establishment of a live vaccine strain against fowl typhoid and paratyphoid
- Protection Against Salmonella Typhimurium, Salmonella Gallinarum, and Salmonella Enteritidis Infection in Layer Chickens Conferred by a Live Attenuated Salmonella Typhimurium Strain
- Evaluation of systemic and mucosal immune responses in mice administered with recombinant Salmonella Typhimurium expressing IutA protein
- Protective efficacy of a high-growth reassortant swine H3N2 inactivated vaccine constructed by reverse genetic manipulation
- Effect of immunization routes and protective efficacy of Brucella antigens delivered via Salmonella vector vaccine