J Vet Sci.
2018 May;19(3):416-425. 10.4142/jvs.2018.19.3.416.
Effect of immunization routes and protective efficacy of Brucella antigens delivered via Salmonella vector vaccine
- Affiliations
-
- 1College of Veterinary Medicine, Chonbuk National University, Iksan 54596, Korea. johnhlee@jbnu.ac.kr
- KMID: 2412132
- DOI: http://doi.org/10.4142/jvs.2018.19.3.416
Abstract
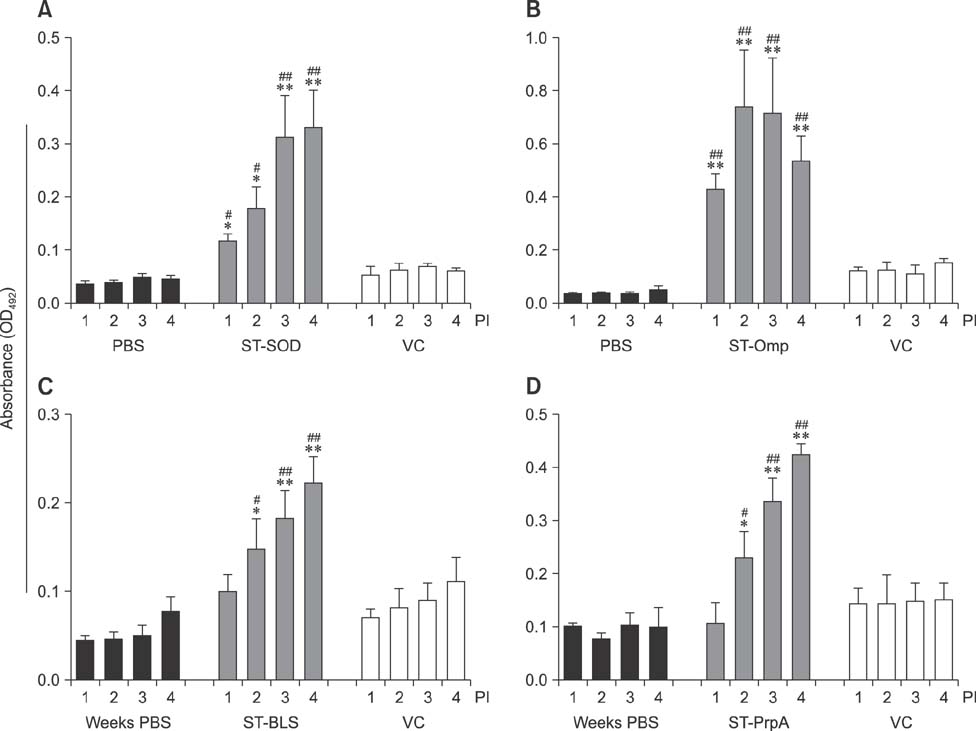
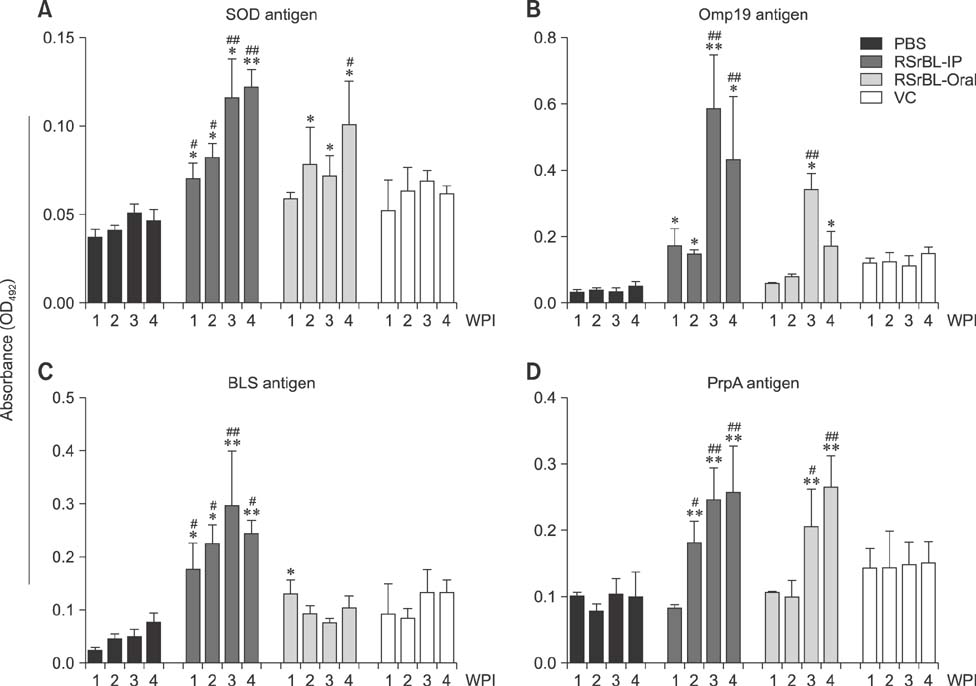
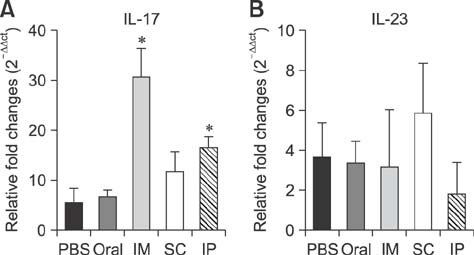
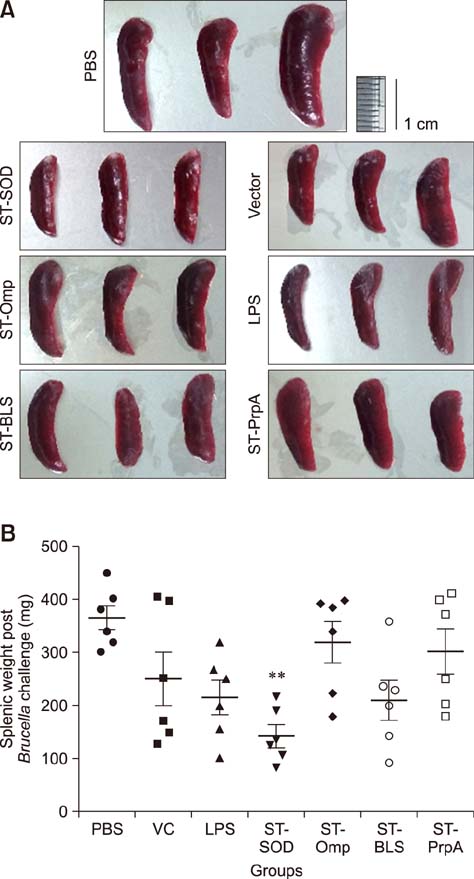
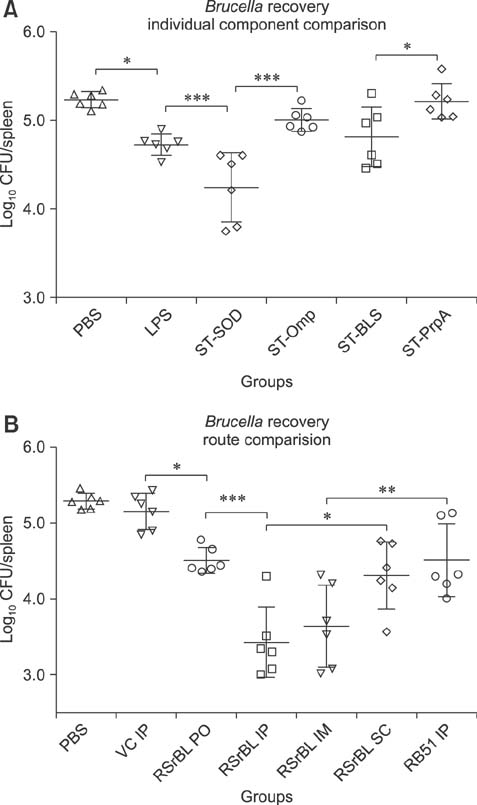
- An anti-Brucella vaccine candidate comprised of purified Brucella lipopolysaccharide (LPS) and a cocktail of four Salmonella Typhimurium (ST)-Brucella vectors was reported previously. Each vector constitutively expressed highly conserved Brucella antigens (rB), viz., lumazine synthase (BLS), proline racemase subunit A, outer membrane protein-19 (Omp19), and Cu-Zn superoxide dismutase (SOD). The present study determined a relative level of protection conferred by each single strain. Upon virulent challenge, the challenge strain was recovered most abundantly in non-immunized control mice, with the ST-Omp19-, ST-BLS-, LPS-, and ST-SOD-immunized mice showing much less burden. Indirect enzyme-linked immunosorbent assay-based assay also confirmed the induction of antigen-specific immunoglobulin G for each antigen delivered. In a route-wise comparison of the combined vaccine candidate, intraperitoneal (IP), intramuscular (IM), and subcutaneous immunizations revealed an indication of highly efficient routes of protection. Splenocytes of mice immunized via IM and IP routes showed significant relative expression of IL-17 upon antigenic pulsing. Taken together, each of the Brucella antigens delivered by ST successfully induced an antigen-specific immune response, and it was also evident that an individual antigen strain can confer a considerable degree of protection. More effective protection was observed when the candidate was inoculated via IP and IM routes.
MeSH Terms
Figure
Reference
-
1. Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O'Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006; 103:8137–8142.
Article2. Corbeil LB, Blau K, Inzana TJ, Nielsen KH, Jacobson RH, Corbeil RR, Winter AJ. Killing of Brucella abortus by bovine serum. Infect Immun. 1988; 56:3251–3261.3. Corbel MJ. Brucellosis in Humans and Animals. Geneva: World Health Organization;2006.4. Han Y, Han X, Wang S, Meng Q, Zhang Y, Ding C, Yu S. The waaL gene is involved in lipopolysaccharide synthesis and plays a role on the bacterial pathogenesis of avian pathogenic Escherichia coli. Vet Microbiol. 2014; 172:486–491.
Article5. International Office of Epizootics (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals: Mammals, Birds, and Bees. 7th ed. Paris: OIE;2009.6. Kim WK, Moon JY, Kim S, Hur J. Comparison between immunization routes of live attenuated Salmonella Typhimurium strains expressing BCSP, Omp3b, and SOD of Brucella abortus in murine model. Front Microbiol. 2016; 7:550.
Article7. Lalsiamthara J, Kamble NM, Lee JH. A live attenuated Salmonella Enteritidis secreting detoxified heat labile toxin enhances mucosal immunity and confers protection against wild-type challenge in chickens. Vet Res. 2016; 47:60.
Article8. Lalsiamthara J, Lee JH. Brucella lipopolysaccharide reinforced Salmonella delivering Brucella immunogens protects mice against virulent challenge. Vet Microbiol. 2017; 205:84–91.
Article9. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001; 25:402–408.
Article10. McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008; 28:445–453.
Article11. Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol. 2008; 38:2636–2649.
Article12. Nymo IH, Tryland M, Godfroid J. A review of Brucella infection in marine mammals, with special emphasis on Brucella pinnipedialis in the hooded seal (Cystophora cristata). Vet Res. 2011; 42:93.
Article13. Oñate AA, Vemulapalli R, Andrews E, Schurig GG, Boyle S, Folch H. Vaccination with live Escherichia coli expressing Brucella abortus Cu/Zn superoxide dismutase protects mice against virulent B. abortus. Infect Immun. 1999; 67:986–988.
Article14. Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005; 352:2325–2336.
Article15. Pasquevich KA, Ibañez AE, Coria LM, García Samartino C, Estein SM, Zwerdling A, Barrionuevo P, Oliveira FS, Seither C, Warzecha H, Oliveira SC, Giambartolomei GH, Cassataro J. An oral vaccine based on U-Omp19 induces protection against B. abortus mucosal challenge by inducing an adaptive IL-17 immune response in mice. PLoS One. 2011; 6:e16203.16. Schurig GG, Roop RM 2nd, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet Microbiol. 1991; 28:171–188.
Article17. Skyberg JA, Thornburg T, Pascual DW. IL-17 is required for protective immunity to nasal Brucella infections in an IFN-γ-dependent fashion. J Immunol. 2009; 182:1 Suppl. 39.23.18. Spera JM, Ugalde JE, Mucci J, Comerci DJ, Ugalde RA. A B lymphocyte mitogen is a Brucella abortus virulence factor required for persistent infection. Proc Natl Acad Sci U S A. 2006; 103:16514–16519.
Article19. Trautwein-Weidner K, Gladiator A, Nur S, Diethelm P, LeibundGut-Landmann S. IL-17-mediated antifungal defense in the oral mucosa is independent of neutrophils. Mucosal Immunol. 2015; 8:221–231.
Article20. Velikovsky CA, Cassataro J, Giambartolomei GH, Goldbaum FA, Estein S, Bowden RA, Bruno L, Fossati CA, Spitz M. A DNA vaccine encoding lumazine synthase from Brucella abortus induces protective immunity in BALB/c mice. Infect Immun. 2002; 70:2507–2511.
Article21. Vitry MA, De Trez C, Goriely S, Dumoutier L, Akira S, Ryffel B, Carlier Y, Letesson JJ, Muraille E. Crucial role of gamma interferon-producing CD4+ Th1 cells but dispensable function of CD8+ T cell, B cell, Th, and Th17 responses in the control of Brucella melitensis infection in mice. Infect Immun. 2012; 80:4271–4280.
Article22. Vitry MA, Hanot Mambres D, De Trez C, Akira S, Ryffel B, Letesson JJ, Muraille E. Humoral immunity and CD4+ Th1 cells are both necessary for a fully protective immune response upon secondary infection with Brucella melitensis. J Immunol. 2014; 192:3740–3752.
Article23. Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001; 194:519–527.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vaccination of goats with a combination Salmonella vector expressing four Brucella antigens (BLS, PrpA, Omp19, and SOD) confers protection against Brucella abortus infection
- Protective efficacy of attenuated Salmonella Typhimurium strain expressing BLS, Omp19, PrpA, or SOD of Brucella abortus in goats
- Development and trial of vaccines against Brucella
- Evaluation of systemic and mucosal immune responses in mice administered with recombinant Salmonella Typhimurium expressing IutA protein
- Hens immunized with live attenuated Salmonella strains expressing virulence-associated genes in avian pathogenic Escherichia coli passively transfer maternal antibodies to chicks