J Vet Sci.
2018 Sep;19(5):643-652. 10.4142/jvs.2018.19.5.643.
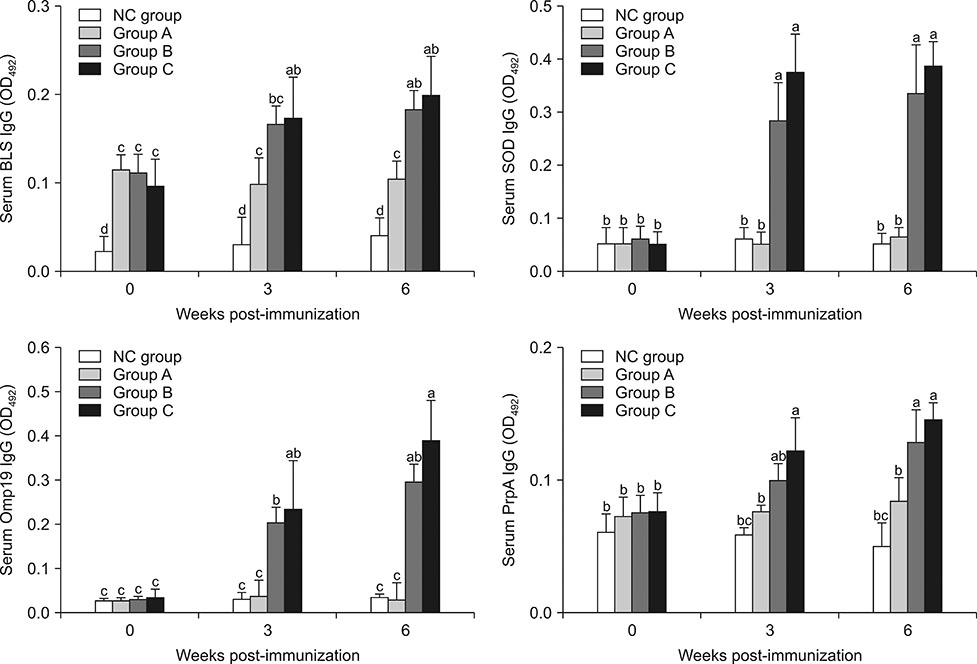
Vaccination of goats with a combination Salmonella vector expressing four Brucella antigens (BLS, PrpA, Omp19, and SOD) confers protection against Brucella abortus infection
- Affiliations
-
- 1College of Veterinary Medicine, Chonbuk National University, Iksan 54596, Korea. bskims@jbnu.ac.kr
- 2Korea Zoonosis Research Institute, Chonbuk National University, Iksan 54596, Korea.
- KMID: 2420933
- DOI: http://doi.org/10.4142/jvs.2018.19.5.643
Abstract
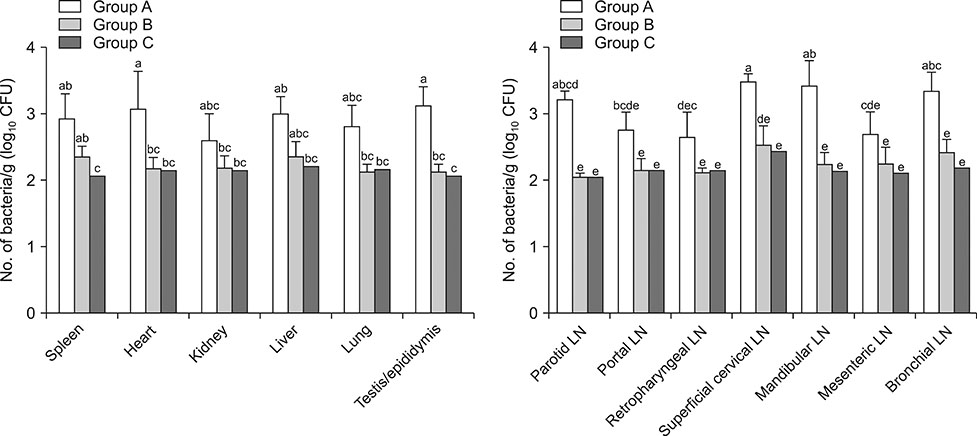
- Salmonella is an intracellular pathogen with a cellular infection mechanism similar to that of Brucella, making it a suitable choice for use in an anti-Brucella immune boost system. This study explores the efficacy of a Salmonella Typhimurium delivery-based combination vaccine for four heterologous Brucella antigens (Brucella lumazine synthase, proline racemase subunit A, outer-membrane protein 19, and Cu/Zn superoxide dismutase) targeting brucellosis in goats. We inoculated the attenuated Salmonella delivery-based vaccine combination subcutaneously at two different inoculation levels; 5 × 10â¹ colony-forming unit (CFU)/mL (Group B) and 5 × 10¹â° CFU/mL (Group C) and challenged the inoculations with virulent Brucella abortus at 6 weeks post-immunization. Serum immunoglobulin G titers against individual antigens in Salmonella immunized goats (Group C) were significantly higher than those of the non-immunized goats (Group A) at 3 and 6 weeks after vaccination. Upon antigenic stimulation, interferon-γ from peripheral blood mononuclear cells was significantly elevated in Groups B and C compared to that in Group A. The immunized goats had a significantly higher level of protection as demonstrated by the low bacterial loads in most tissues from the goats challenged with B. abortus. Relative real-time polymerase chain reaction results revealed that the expression of Brucella antigens was lower in spleen, kidney, and lung of immunized goats than of non-immunized animals. Also, treatment with our combination vaccine ameliorated histopathological lesions induced by the Brucella infection. Overall, the Salmonella Typhimurium delivery-based combination vaccine was effective in delivering immunogenic Brucella proteins, making it potentially useful in protecting livestock from brucellosis.
MeSH Terms
Figure
Reference
-
1. Adone R, Francia M, Pistoia C, Petrucci P, Pesciaroli M, Pasquali P. Protective role of antibodies induced by Brucella melitensis B115 against B. melitensis and Brucella abortus infections in mice. Vaccine. 2012; 30:3992–3995.
Article2. Bancroft JD, Stevens A, Turner DR. Theory and Practice of Histological Techniques. 4th ed. New York: Churchill Livingstone;1996. p. 83–121.3. Corbel MJ. Brucellosis: an overview. Emerg Infect Dis. 1997; 3:213–221.
Article4. Friberg D, Bryant J, Shannon W, Whiteside TL. In vitro cytokine production by normal human peripheral blood mononuclear cells as a measure of immunocompetence or the state of activation. Clin Diagn Lab Immunol. 1994; 1:261–268.
Article5. Haag AF, Myka KK, Arnold MF, Caro-Hernández P, Ferguson GP. Importance of lipopolysaccharide and cyclic β-1,2-glucans in Brucella-mammalian infections. Int J Microbiol. 2010; 2010:124509.6. Hur J, Lee JH. Enhancement of immune responses by an attenuated Salmonella enterica serovar Typhimurium strain secreting an Escherichia coli heat-labile enterotoxin B subunit protein as an adjuvant for a live Salmonella vaccine candidate. Clin Vaccine Immunol. 2011; 18:203–209.
Article7. Jacques I, Cloeckaert A, Limet JN, Dubray G. Protection conferred on mice by combinations of monoclonal antibodies directed against outer-membrane proteins or smooth lipopolysaccharide of Brucella. J Med Microbiol. 1992; 37:100–103.
Article8. Jubb KVF, Kennedy PC, Palmer N. Pathology of Domestic Animals. 3rd ed. San Diego: Academic Press;1985. Vol. 2:p. 234–236.9. Kim WK, Moon JY, Kim S, Hur J. Comparison between immunization routes of live attenuated Salmonella Typhimurium strains expressing BCSP31, Omp3b, and SOD of Brucella abortus in murine model. Front Microbiol. 2016; 7:550.
Article10. Lalsiamthara J, Gogia N, Goswami TK, Singh RK, Chaudhuri P. Intermediate rough Brucella abortus S19Δper mutant is DIVA enable, safe to pregnant guinea pigs and confers protection to mice. Vaccine. 2015; 33:2577–2583.
Article11. Lalsiamthara J, Lee JH. Brucella lipopolysaccharide reinforced Salmonella delivering Brucella immunogens protects mice against virulent challenge. Vet Microbiol. 2017; 205:84–91.
Article12. Lee JJ, Lim JJ, Kim DG, Simborio HL, Kim DH, Reyes AW, Min W, Lee HJ, Kim DH, Chang HH, Kim S. Characterization of culture supernatant proteins from Brucella abortus and its protection effects against murine brucellosis. Comp Immunol Microbiol Infect Dis. 2014; 37:221–228.
Article13. Muñoz-Montesino C, Andrews E, Rivers R, González-Smith A, Moraga-Cid G, Folch H, Céspedes S, Oñate AA. Intraspleen delivery of a DNA vaccine coding for superoxide dismutase (SOD) of Brucella abortus induces SOD-specific CD4+ and CD8+ T cells. Infect Immun. 2004; 72:2081–2087.
Article14. Newby DT, Hadfield TL, Roberto FF. Real-time PCR detection of Brucella abortus: a comparative study of SYBR green I, 5′-exonuclease, and hybridization probe assays. Appl Environ Microbiol. 2003; 69:4753–4759.
Article15. O'Leary S, Sheahan M, Sweeney T. Brucella abortus detection by PCR assay in blood, milk and lymph tissue of serologically positive cows. Res Vet Sci. 2006; 81:170–176.16. Pak DK, Lee CW. The first brucellosis of cattle in Korea. J Korean Vet Med Assoc. 1959; 3:392–395.17. Park MS, Woo YS, Lee MJ, Shim SK, Lee HK, Choi YS, Lee WH, Kim KH, Park MY. The first case of human brucellosis in Korea. Infect Chemother. 2003; 35:461–466.18. Poester FP, Gonçalves VS, Paixão TA, Santos RL, Olsen SC, Schurig GG, Lage AP. Efficacy of strain RB51 vaccine in heifers against experimental brucellosis. Vaccine. 2006; 24:5327–5334.
Article19. Redkar R, Rose S, Bricker B, DelVecchio V. Real-time detection of Brucella abortus, Brucella melitensis and Brucella suis. Mol Cell Probes. 2001; 15:43–52.
Article20. Rueckert C, Guzmán CA. Vaccines: from empirical development to rational design. PLoS Pathog. 2012; 8:e1003001.
Article21. Tan SY, Davis C. David Bruce (1855–1931): discoverer of brucellosis. Singapore Med J. 2011; 52:138–139.22. Thomas EL, Bracewell CD, Corbel MJ. Characterisation of Brucella abortus strain 19 cultures isolated from vaccinated cattle. Vet Rec. 1981; 108:90–93.
Article23. Wee SH, Nam HM, Kim CH. Emergence of brucellosis in cattle in the Republic of Korea. Vet Rec. 2008; 162:556–557.
Article24. Xavier MN, Winter MG, Spees AM, Nguyen K, Atluri VL, Silva TM, Bäumler AJ, Müller W, Santos RL, Tsolis RM. CD4+ T cell-derived IL-10 promotes Brucella abortus persistence via modulation of macrophage function. PLoS Pathog. 2013; 9:e1003454.25. Yoo SJ, Choi YS, Lim HS, Lee K, Park MY, Chu C, Kang YA. [Seroprevalence and risk factors of brucellosis among slaughterhouse workers in Korea]. J Prev Med Public Health. 2009; 42:237–242. Korean.
Article26. Zachary JF, McGavin MD. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis: Mosby;2012. p. 751–754.27. Zhao Z, Li M, Luo D, Xing L, Wu S, Duan Y, Yang P, Wang X. Protection of mice from Brucella infection by immunization with attenuated Salmonella enterica serovar typhimurium expressing A L7/L12 and BLS fusion antigen of Brucella. Vaccine. 2009; 27:5214–5219.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective efficacy of attenuated Salmonella Typhimurium strain expressing BLS, Omp19, PrpA, or SOD of Brucella abortus in goats
- Effect of immunization routes and protective efficacy of Brucella antigens delivered via Salmonella vector vaccine
- Successful Medical Treatment of Prosthetic Mitral Valve Endocarditis Caused by Brucella abortus
- Diversity of Humoral Immune Responses to Recombinant Proteins of Brucella abortus Among Residents in Cheju Province
- Immunization of BALB/c mice with Brucella abortus 2308DeltawbkA confers protection against wild-type infection