J Bacteriol Virol.
2018 Sep;48(3):93-101. 10.4167/jbv.2018.48.3.93.
IKKγ Facilitates the Activation of NF-κB by Hepatitis C Virus Core Protein
- Affiliations
-
- 1Department of Biohealth Sciences, Changwon National University, Changwon, Korea. dwkim@changwon.ac.kr
- 2Department of Biology and Chemistry, Changwon National University Changwon, Korea.
- KMID: 2421304
- DOI: http://doi.org/10.4167/jbv.2018.48.3.93
Abstract
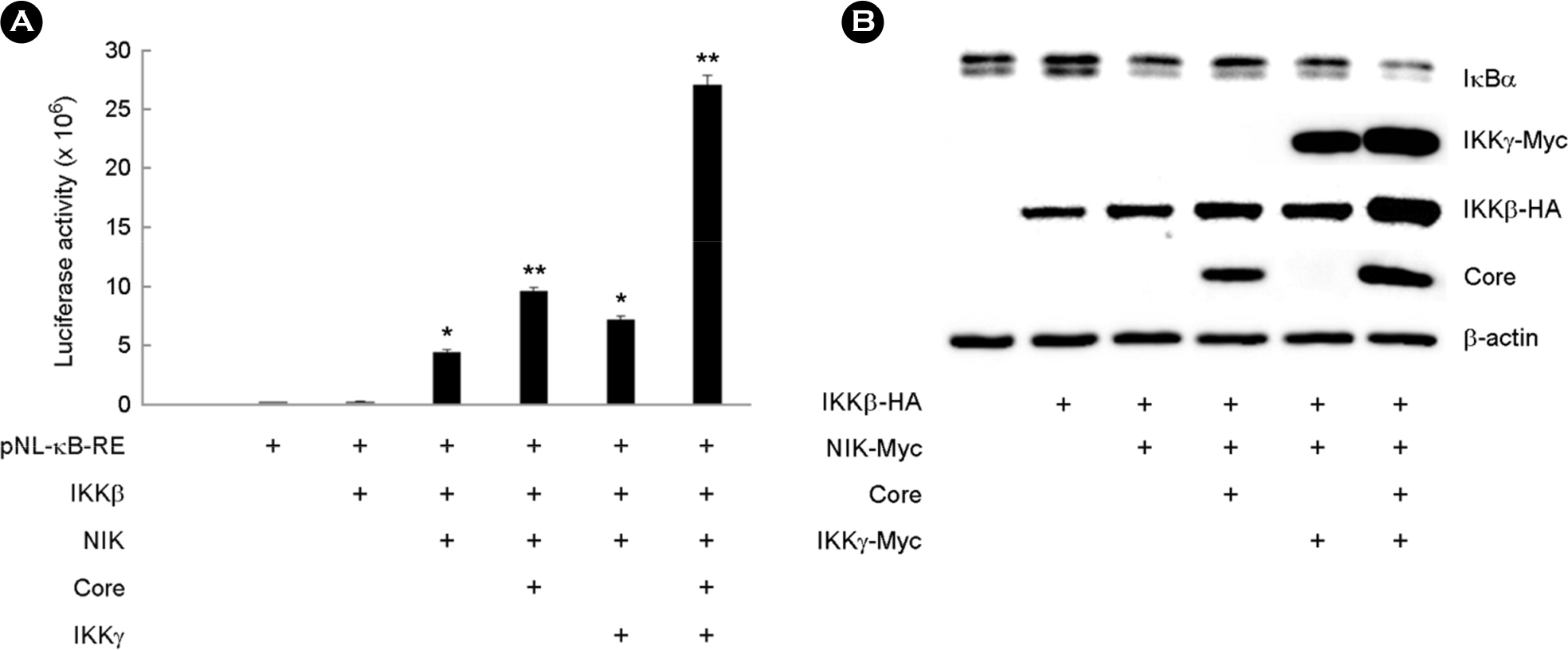
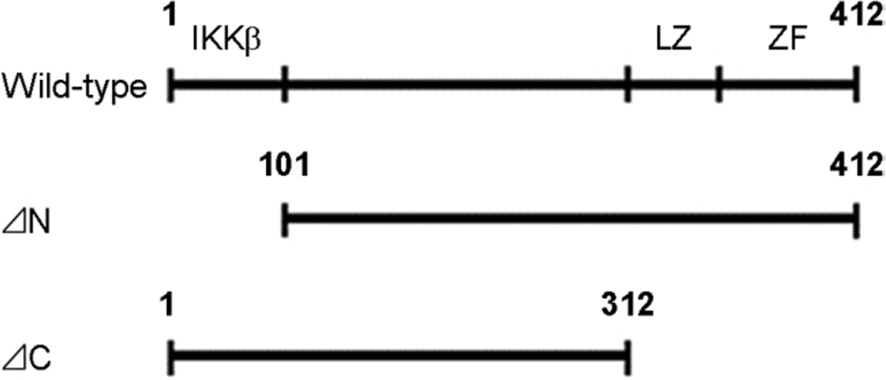
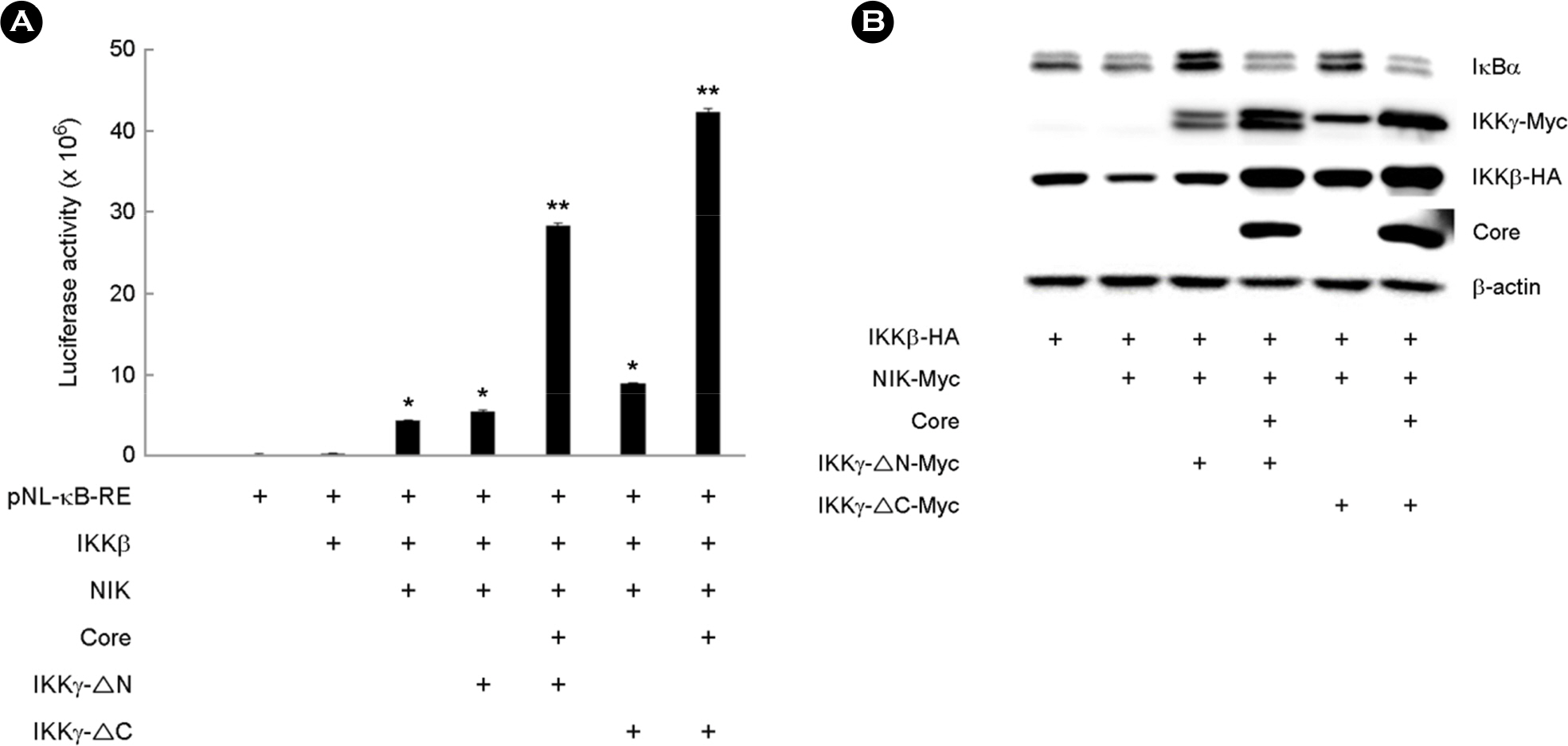
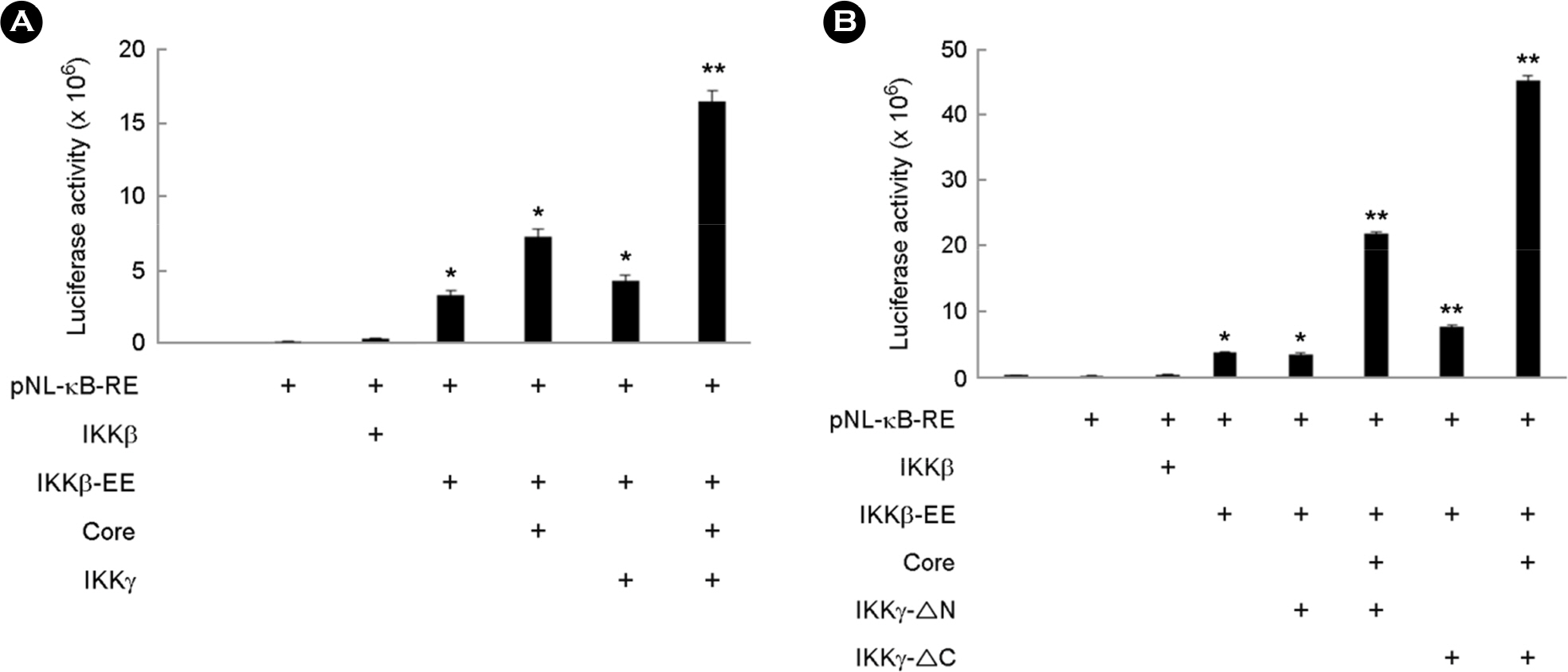
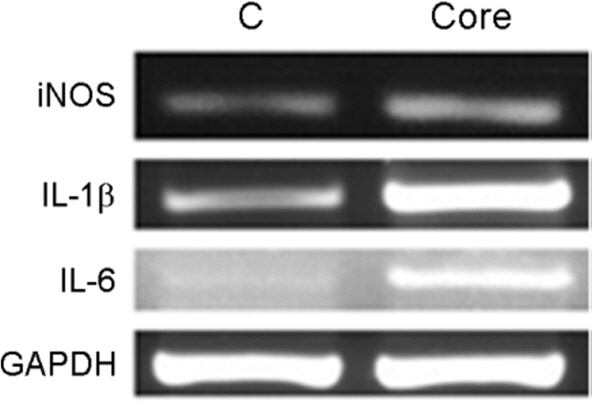
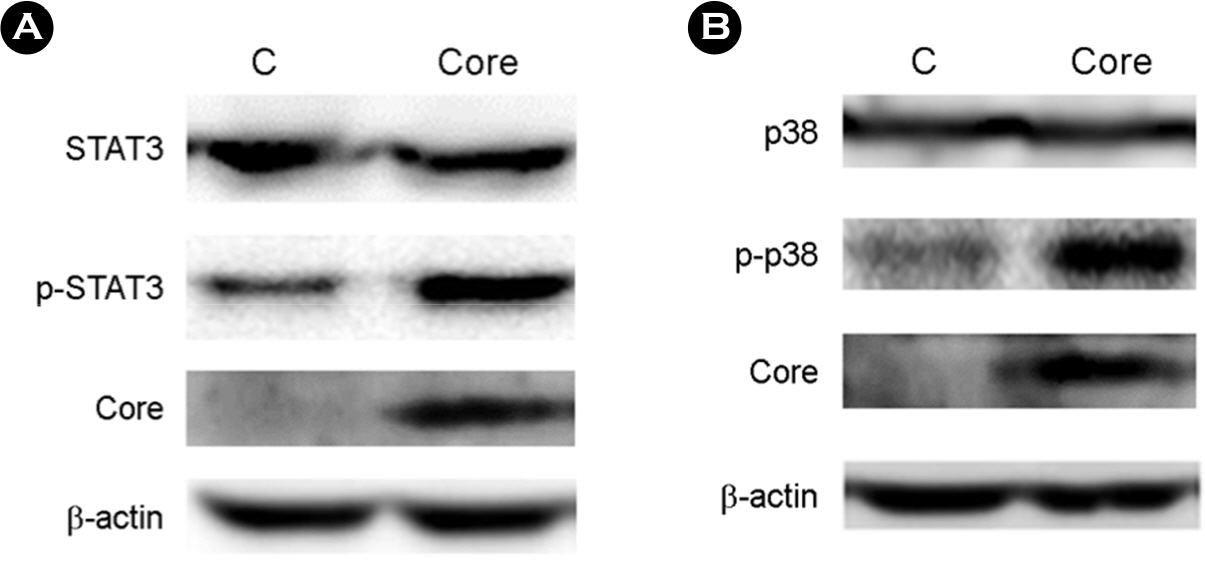
- Hepatitis C virus (HCV) is a major cause of chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. HCV core protein has been shown to modulate various cellular signaling pathways including the nuclear factor κB (NF-κB) pathway which is associated with inflammation, cell proliferation and apoptosis. However, there have been conflicting reports about the effect of HCV core protein on NF-κB pathway, and the mechanism by which the core protein affects NF-κB activity remains nuclear. In this study, the functional interaction of HCV core protein and IκB kinase γ (IKKγ) was investigated using the expression plasmids of core and the components of IKK complex. The data revealed that HCV core protein activates NF-κB. Also, HCV core protein up-regulated the phosphorylation and degradation of IκBα. The activating effect of HCV core protein on NF-κB was synergistically elevated by IKKγ. It was noticed that the N-terminal IKKβ binding site, C-terminal leucine zipper, and zinc finger domains of IKKγ are not necessary for its synergistic effect. HCV core protein and IKKγ appeared to activate NF-κB by up-regulating the IKKβ activity resulting in the degradation of IκBα. As expected, HCV core protein induced the expression of NF-κB-targeted pro-inflammatory genes such as iNOS, IL-1β and IL-6 in the transcription level. These results suggest that HCV core protein induces NF-κB through the interaction with IKKγ and may play a critical role in the development of inflammation and related liver diseases.
Keyword
MeSH Terms
Figure
Reference
-
1). Tanaka K, Hirohata T, Koga S, Sugimachi K, Kanematsu T, Ohryohji F, et al. Hepatitis C and hepatitis B in the etiology of hepatocellular carcinoma in the Japanese population. Cancer Res. 1991; 51:2842–7.2). Grakoui A, Wychowski C, Lin C, Feinstone SM, Rice CM. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993; 67:1385–95.
Article3). Hijikata M, Kato N, Ootsuyama Y, Nakagawa M, Shimotohno K. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci USA. 1991; 88:5547–51.
Article4). Mizushima H, Hijikata M, Tanji Y, Kimura K, Shimotohno K. Analysis of N-terminal processing of hepatitis C virus nonstructural protein 2. J Virol. 1994; 68:2731–4.
Article5). Shrivastava A, Manna SK, Ray R, Aggarwal BB. Ectopic expression of hepatitis C virus core protein differentially regulates nuclear transcription factors. J Virol. 1998; 72:9722–8.
Article6). Yoshida H, Kato N, Shiratori Y, Otsuka M, Maeda S, Kato J, et al. Hepatitis C virus core protein activates nuclear factor κ B-dependent signaling through tumor necrosis factor receptor-associated factor. J Biol Chem. 2001; 276:16399–405.7). Marusawa H, Hijicata M, Chiba T, Shimotohno K. Hepatitis C virus core protein inhibits Fas- and tumor necrosis factor alpha-mediated apoptosis via NF-κB activation. J Virol. 1999; 73:4713–20.
Article8). Zhu N, Ware CF, Lai MM. Hepatitis C virus core protein enhances FADD-mediated apoptosis and suppresses TRADD signaling of tumor necrosis factor receptor. Virology. 2001; 283:178–87.
Article9). Park J, Kang W, Ryu SW, Kim WI, Chang DY, Lee DH, et al. Hepatitis C virus infection enhances TNFα-induced cell death via suppression of NF-κB. Hepatology. 2012; 56:831–40.
Article10). Ray RB, Meyer K, Ray R. Suppression of apoptotic cell death by hepatitis C virus core protein. Virology. 1996; 226:176–82.
Article11). Ghosh G, Wang VY, Huang DB, Fusco A. NF-κB regulation: lessons from structures. Immunol Rev. 2012; 246:36–58.
Article12). Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008; 132:344–62.
Article13). Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011; 12:695–708.
Article14). Ghosh S, Febin Prabhu Dass J. Non-canonical pathway network modelling and ubiquitination site prediction through homology modelling of NF-κB. Gene. 2016; 581:48–56.
Article15). Hinz M, Arslan SC, Scheidereit C. It takes two to tango: IκBs, the multifunctional partners of NF-κB. Immunol Rev. 2012; 246:59–76.
Article16). Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011; 21:103–15.
Article17). Mauro C, Pacifico F, Lavorgna A, Mellone S, Iannetti A, Acquaviva R, et al. ABIN-1 binds to NEMO/IKKγ and co-operates with A20 in inhibiting NF-κB. J Biol Chem. 2006; 281:18482–8.
Article18). Yamamoto Y, Kim DW, Kwak YT, Parajapati S, Verma U, Gaynor RB. IKKγ/NEMO facilitates the recruitment of the IκB proteins into the IκB kinase complex. J Biol Chem. 2001; 276:36327–36.
Article19). Kwon WJ, Kim SH, Park YO, Cho M, Kang CD, Lee G, et al. IKKγ inhibits activation of NF-κB by NIK. Mol cells. 2004; 18:200–6.20). Li XH, Fang X, Gaynor RB. Role of Ikkγ/NEMO in assembly of the ikappa B kinase complex. J Biol Chem. 2001; 276:4494–500.21). Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, et al. Ikk-1 and Ikk-2: Cytokine-activated ikappa B kinase essential for NF-κB activation. Science. 1997:278:860–6.22). Tang H, Chen H, Jia Y, Liu X, Han Z, Wang A, et al. Effect of inhibitors of endocytosis and NF-κB signal pathway on folate-conjugated nanoparticle endocytosis by rat Kupffer cells. Int J Nanomedicine. 2017; 12:6937–47.23). He G, Karin M. NF-κB and STAT3 – key players in liver inflammation and cancer. Cell Res. 2011; 21:159–68.
Article24). Craig R, Larkin A, Mingo AM, Thuerauf DJ, Andrews C, McDonough PM, et al. p38 MAPK and NF-κB collaborate to induce interleukin-6 gene expression and release. J Biol Chem. 2000; 275:23814–24.
Article25). Bouffard P, Hayashi PH, Acevedo R, Levy N, Zeldis JB. Hepatitis C virus is detected in a monocyte/macrophage subpopulation of peripheral blood mononuclear cells of infected patients. J Infect Dis. 1992; 166:1276–80.
Article26). Heydtmann M, Adams DH. Chemokines in the immunopathogenesis of hepatitis C infection. Hepatology. 2009; 49:676–88.
Article27). Saito K, Meyer K, Warner R, Basu A, Ray RB, Ray R. Hepatitis C virus core protein inhibits tumor necrosis factor alpha-mediated apoptosis by a protective effect involving cellular FLICE inhibitory protein. J virol. 2006; 80:4372–9.
Article28). McLauchlan J. Properties of the hepatitis C virus core protein: a structural protein that modulates cellular processes. J viral hepat. 2000; 7:2–14.
Article29). Bühler S, Bartenschlager R. Promotion of hepatocellular carcinoma by hepatitis C virus. Dig Dis. 2012; 30:445–52.
Article30). Hayden MS, Ghosh S. NF-κB in immunology. Cell Res. 2011; 21:223–44.31). Xing Y, Wang X, Jameson SC, Hogquist KA. Late stage of T cell maturation in the thymus involve NF-κB and tonic type I interferon signaling. Nat Immunol. 2016; 17:565–73.32). Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009; 30:1073–81.
Article33). Bours V, Bentires-Alj M, Hellin AC, Viatour P, Robe P, Delhalle S, et al. Nuclear factor-kappa B, cancer, and apoptosis. Biochem Pharmacol. 2000; 60:1085–9.34). Wu JT, Kral JG. The NF-kappaB/IkappaB signaling system: A molecular target in breast cancer therapy. J Surg Res. 2005; 123:158–69.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Analysis for Basal Activation of NF-κB in Acral Lentiginous Melanoma
- Controls of Nuclear Factor-Kappa B Signaling Activity by 5’-AMP-Activated Protein Kinase Activation With Examples in Human Bladder Cancer Cells
- Induction of Interleukin-8 Expression in Synovial Cell by Hepatitis C Virus Core Protein
- Prevention of Viral Hepatitis and Vaccination
- New biomarkers of hepatitis B virus (HBV) infection: HBV RNA and HBV core-related antigen, new kids on the block?