Ann Hepatobiliary Pancreat Surg.
2018 Aug;22(3):179-184. 10.14701/ahbps.2018.22.3.179.
Synergistic effect of metformin on sorafenib in in vitro study using hepatocellular carcinoma cell lines
- Affiliations
-
- 1Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. shwang@amc.seoul.kr
- 2Asan Institute of Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2420606
- DOI: http://doi.org/10.14701/ahbps.2018.22.3.179
Abstract
- BACKGROUNDS/AIMS
Hepatocellular carcinoma (HCC) recurrence remains a great concern following hepatic resection and liver transplantation. We investigated the metformin-induced cytotoxic effects on sorafenib in an in vitro study using HCC cell lines.
METHODS
This research was conducted through an in vitro study using one HepG2.2.15 liver tumor and two patient-derived graft HCC cell lines.
RESULTS
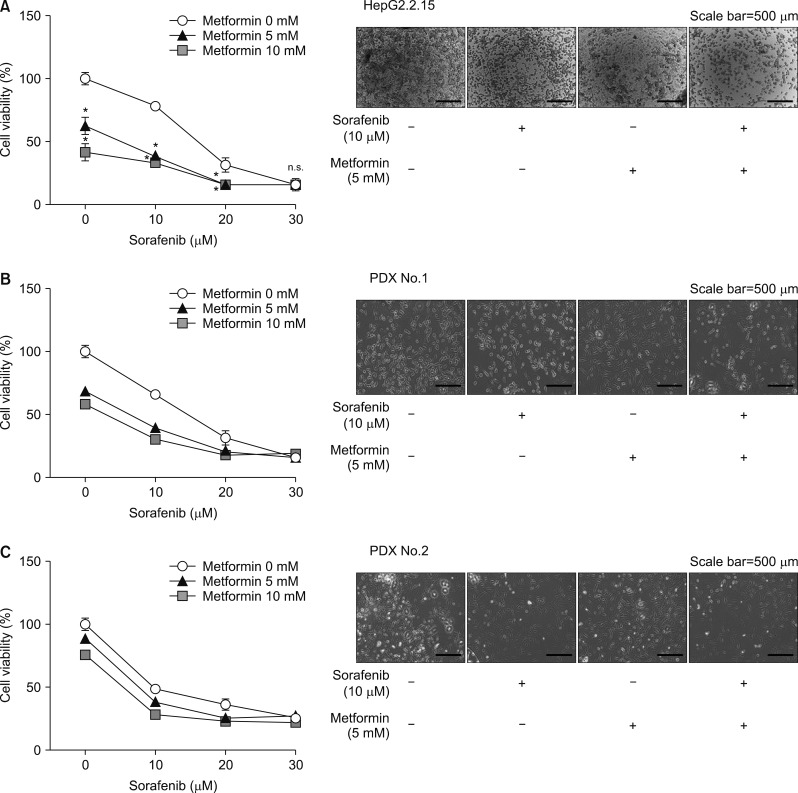
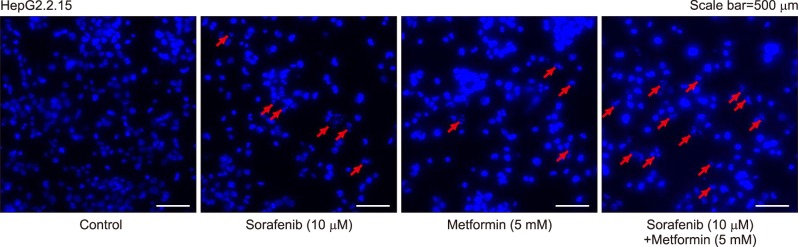
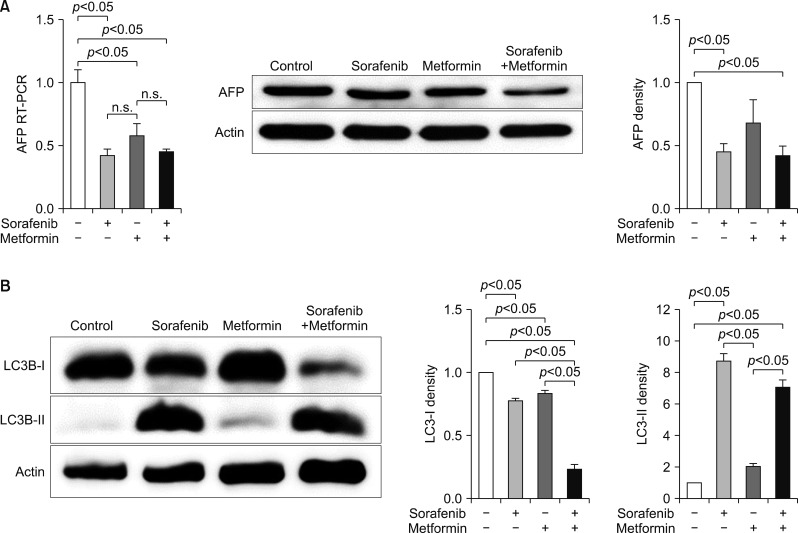
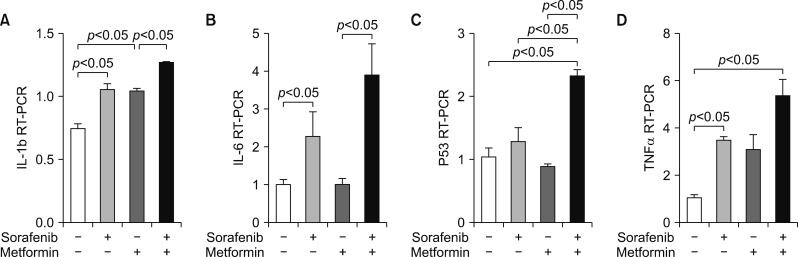
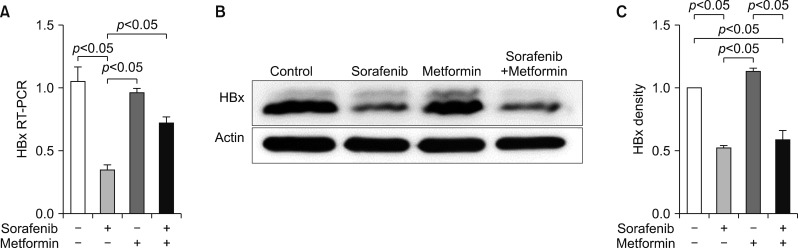
An in vitro study revealed noticeable cytotoxic effects of metformin as well as noticeable synergistic cytotoxic effects of metformin and sorafenib on cell viability. Assays for the mechanisms of action of antitumor effects revealed that alpha-fetoprotein expression was suppressed by both metformin and sorafenib, but no synergistic effect was observed. LC3-I and LC3-II assays revealed the synergistic upregulation of autophagy and assays for IL-1β, IL-6, p53, and TNF-α revealed the synergistic upregulation of cell damage and apoptosis. In contrast, metformin did not affect HBx expression, thus no noticeable synergistic effect was considered to be present.
CONCLUSIONS
Our in vitro study demonstrated cytotoxic effects of metformin and synergistic antitumor effects of sorafenib. These results should be verified in further clinical studies with patients of advanced HCC.
Keyword
MeSH Terms
Figure
Reference
-
1. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390. PMID: 18650514.
Article2. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim J, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34. PMID: 19095497.
Article3. Davis E, Wiesner R, Valdecasas J, Kita Y, Rossi M, Schwartz M. Treatment of recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2011; 17(Suppl 2):S162–S166. PMID: 21688382.
Article4. Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011; 71:4366–4372. PMID: 21540236.
Article5. Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007; 67:6745–6752. PMID: 17638885.
Article6. Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007; 67:10804–10812. PMID: 18006825.
Article7. Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006; 66:10269–10273. PMID: 17062558.
Article8. Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013; 62:606–615. PMID: 22773548.
Article9. Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010; 30:750–758. PMID: 20331505.
Article10. Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012; 107:46–52. PMID: 22085817.
Article11. Kang WH, Tak E, Hwang S, Song GW, Jwa E, Lee YJ, et al. Metformin-associated chemopreventive effects on recurrence after hepatic resection of hepatocellular carcinoma: from in vitro to a clinical study. Anticancer Res. 2018; 38:2399–2407. PMID: 29599368.
Article12. Ling S, Song L, Fan N, Feng T, Liu L, Yang X, et al. Combination of metformin and sorafenib suppresses proliferation and induces autophagy of hepatocellular carcinoma via targeting the mTOR pathway. Int J Oncol. 2017; 50:297–309. PMID: 27959383.
Article13. Cheung PF, Yip CW, Ng LW, Lo KW, Chow C, Chan KF, et al. Comprehensive characterization of the patient-derived xenograft and the paralleled primary hepatocellular carcinoma cell line. Cancer Cell Int. 2016; 16:41. PMID: 27279800.
Article14. Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010; 11:390–401. PMID: 20444419.
Article15. Tak E, Hwang S, Lee HC, Ko GY, Ahn CS, Yoon YI, et al. Apoptosis of hepatitis b virus-expressing liver tumor cells induced by a high concentration of nucleos(t)ide analogue. Anticancer Res. 2016; 36:6059–6069. PMID: 27793933.
Article16. Ha TY, Hwang S, Moon KM, Won YJ, Song GW, Kim N, et al. Sorafenib inhibits migration and invasion of hepatocellular carcinoma cells through suppression of matrix metalloproteinase expression. Anticancer Res. 2015; 35:1967–1976. PMID: 25862849.17. Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med. 2007; 13:433–442. PMID: 17905659.
Article18. Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012; 22:820–827. PMID: 22722338.19. Groenendijk FH, Mellema WW, van der Burg E, Schut E, Hauptmann M, Horlings HM, et al. Sorafenib synergizes with metformin in NSCLC through AMPK pathway activation. Int J Cancer. 2015; 136:1434–1444. PMID: 25080865.
Article20. Casadei Gardini A, Marisi G, Scarpi E, Scartozzi M, Faloppi L, Silvestris N, et al. Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib. Expert Opin Pharmacother. 2015; 16:2719–2725. PMID: 26513009.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metformin and statins and their role in reducing hepatocellular carcinoma risk: Randomized trials are needed: Editorial on “Metformin and statins reduce hepatocellular carcinoma risk in chronic hepatitis C patients with failed antiviral therapy”
- Treatments Other than Sorafenib for Patients with Advanced Hepatocellular Carcinoma
- Treatment options after sorafenib failure in patients with hepatocellular carcinoma
- Absence of antitumor effects of metformin in sorafenib-treated patients with hepatocellular carcinoma recurrence after hepatic resection and liver transplantation
- The Efficacy of Sorafenib for Patients With Advanced Hepatocellular Carcinoma