Ann Hepatobiliary Pancreat Surg.
2018 Nov;22(4):297-304. 10.14701/ahbps.2018.22.4.297.
Absence of antitumor effects of metformin in sorafenib-treated patients with hepatocellular carcinoma recurrence after hepatic resection and liver transplantation
- Affiliations
-
- 1Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. shwang@amc.seoul.kr
- 2Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2427359
- DOI: http://doi.org/10.14701/ahbps.2018.22.4.297
Abstract
- BACKGROUNDS/AIMS
Hepatocellular carcinoma (HCC) recurrence following hepatic resection (HR) and liver transplantation (LT) remains a great concern. We assessed the antitumor effects of metformin in patients treated with sorafenib for HCC recurrence after HR or LT.
METHODS
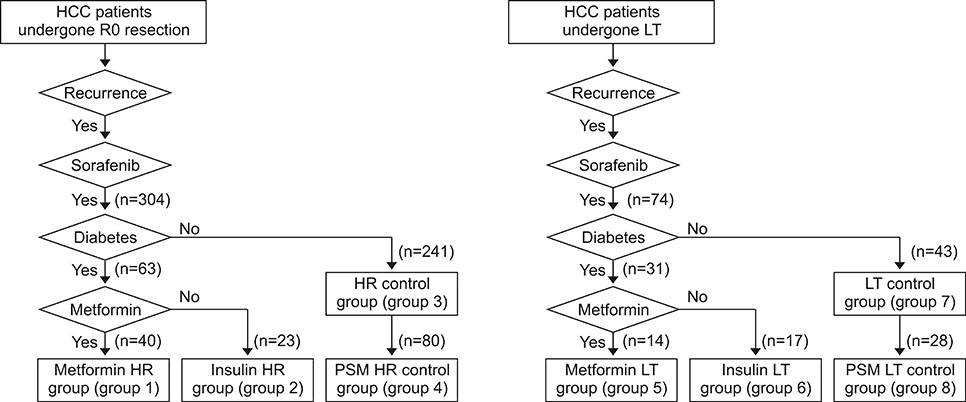
The two clinical retrospective studies involved metformin therapy of 304 HR patients and 74 LT recipients who were treated with sorafenib.
RESULTS
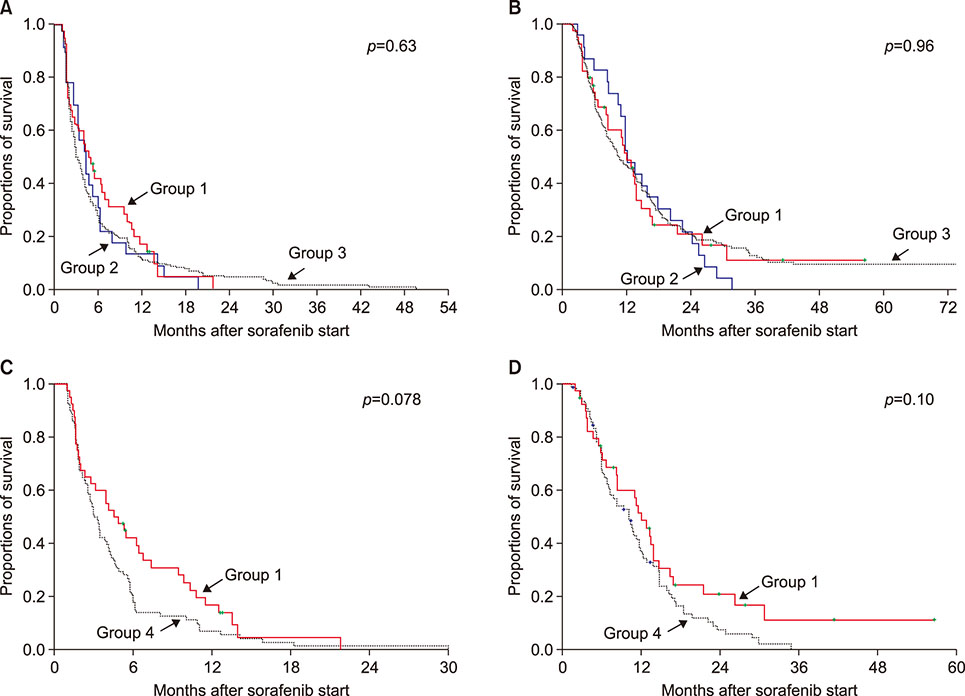
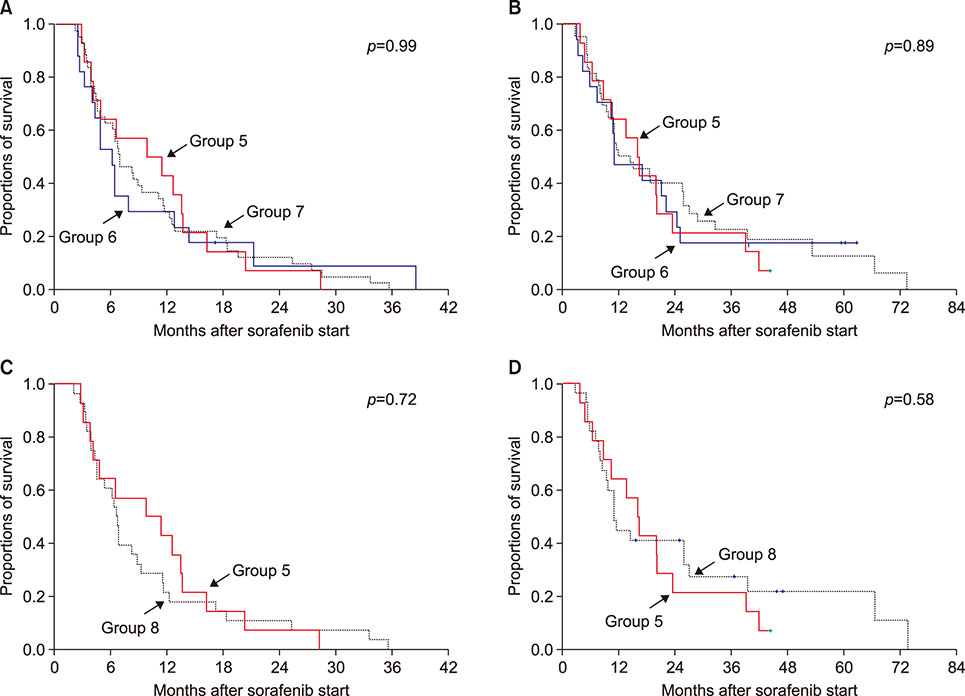
In the study involving patients who had undergone HR, death occurred in 245 of the 304 patients (80.6%) during a median follow-up of 10.2 months after sorafenib administration. The metformin HR group (group 1; n=40) showed no prognostic difference in progression-free and overall survival rates compared with the all-HR control group (group 3; n=241) and propensity score-matched HR control group (group 4; n=80). In the clinical study of recipients exposed to LT, death occurred in 62 of the 74 patients (83.8%) during a median follow-up of 13.6 months (range: 3-76 months) after sorafenib administration. The metformin LT group (group 5; n=14) showed no prognostic difference in progression-free and overall survival rates compared with the all-LT control group (group 7; n=43) and propensity score-matched LT control group (group 8; n=28).
CONCLUSIONS
Our clinical studies demonstrated absence of synergistic antitumor effects of metformin. Further high-volume studies are necessary to assess the role of metformin in patients treated with sorafenib for advanced HCC.
Keyword
MeSH Terms
Figure
Reference
-
1. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.
Article2. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.
Article3. Davis E, Wiesner R, Valdecasas J, Kita Y, Rossi M, Schwartz M. Treatment of recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2011; 17:Suppl 2. S162–S166.
Article4. Ha TY, Hwang S, Hong HN, Choi YI, Yoon SY, Won YJ, et al. Synergistic effect of sorafenib and vitamin K on suppression of hepatocellular carcinoma cell migration and metastasis. Anticancer Res. 2015; 35:1985–1995.5. Jung DH, Hwang S, Song GW, Ryoo BY, Kim N, Tak E, et al. An interim safety analysis of hepatocellular carcinoma patients administrating oral vitamin K with or without sorafenib. Korean J Hepatobiliary Pancreat Surg. 2015; 19:1–5.
Article6. Yoshida H, Shiratori Y, Kudo M, Shiina S, Mizuta T, Kojiro M, et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology. 2011; 54:532–540.
Article7. Zhong JH, Mo XS, Xiang BD, Yuan WP, Jiang JF, Xie GS, et al. Postoperative use of the chemopreventive vitamin K2 analog in patients with hepatocellular carcinoma. PLoS One. 2013; 8:e58082.
Article8. Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014; 312:57–67.9. Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011; 71:4366–4372.
Article10. Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007; 67:6745–6752.
Article11. Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007; 67:10804–10812.
Article12. Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006; 66:10269–10273.
Article13. Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013; 62:606–615.
Article14. Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010; 30:750–758.
Article15. Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012; 107:46–52.
Article16. Ling S, Song L, Fan N, Feng T, Liu L, Yang X, et al. Combination of metformin and sorafenib suppresses proliferation and induces autophagy of hepatocellular carcinoma via targeting the mTOR pathway. Int J Oncol. 2017; 50:297–309.
Article17. You A, Cao M, Guo Z, Zuo B, Gao J, Zhou H, et al. Metformin sensitizes sorafenib to inhibit postoperative recurrence and metastasis of hepatocellular carcinoma in orthotopic mouse models. J Hematol Oncol. 2016; 9:20.
Article18. Korean Association for the Study of the Liver. KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2016; 22:18–75.19. Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 Patients. J Gastrointest Surg. 2015; 19:1281–1290.
Article20. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60.
Article21. Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med. 2007; 13:433–442.
Article22. Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012; 22:820–827.23. Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010; 11:390–401.
Article24. Groenendijk FH, Mellema WW, van der Burg E, Schut E, Hauptmann M, Horlings HM, et al. Sorafenib synergizes with metformin in NSCLC through AMPK pathway activation. Int J Cancer. 2015; 136:1434–1444.
Article25. Perricone G, Mancuso A, Belli LS, Mazzarelli C, Zavaglia C. Sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation: does mTOR inhibitors association augment toxicity? Eur J Gastroenterol Hepatol. 2014; 26:577–578.26. Casadei Gardini A, Marisi G, Scarpi E, Scartozzi M, Faloppi L, Silvestris N, et al. Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib. Expert Opin Pharmacother. 2015; 16:2719–2725.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synergistic effect of metformin on sorafenib in in vitro study using hepatocellular carcinoma cell lines
- Treatments Other than Sorafenib for Patients with Advanced Hepatocellular Carcinoma
- Liver resection after sorafenib therapy in advanced hepatocellular carcinoma: a case report
- Efficacy of Sorafenib for the Treatment of Post-Transplant Hepatocellular Carcinoma Recurrence
- Surgical Management of Hepatocellular Carcinoma