Ann Lab Med.
2019 Jan;39(1):36-42. 10.3343/alm.2019.39.1.36.
Molecular Epidemiology and Mechanisms of 43 Low-Level Linezolid-Resistant Enterococcus faecalis Strains in Chongqing, China
- Affiliations
-
- 1Department of Clinical Laboratory, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China. xiayun12cn@aliyun.com
- KMID: 2420269
- DOI: http://doi.org/10.3343/alm.2019.39.1.36
Abstract
- BACKGROUND
Enterococcus faecalis strains with low-level resistance to linezolid (an oxazolidinone antibiotic) have become common. No large-scale study has examined the underlying mechanisms in linezolid-resistant E. faecalis (LRE) strains. We investigated these mechanisms and molecular characteristics in Chongqing, China.
METHODS
A total of 1,120 non-duplicated E. faecalis strains collected from August 2014 to June 2017 underwent drug susceptibility testing. LRE strains were screened for optrA, cfr, and mutations in the 23S rRNA and ribosomal proteins L3 and L4 by PCR amplification and sequencing. Multi-locus sequence typing (MLST) and pulsed-field gel electrophoresis (PFGE) were used for epidemiological analysis.
RESULTS
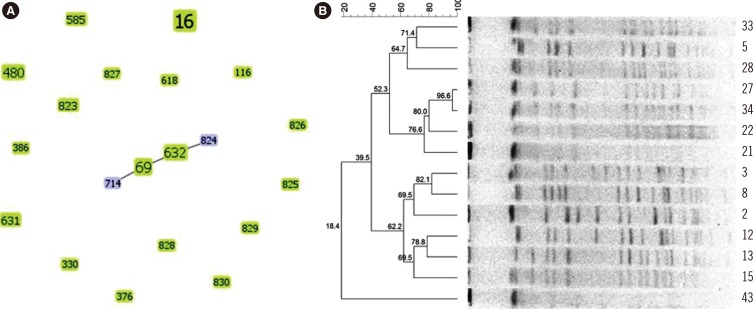
All 43 low-level LRE strains (minimum inhibitory concentration: 8-16 mg/L) harbored optrA; cfr and 23S rRNA mutations were not detected. Novel mutations in the ribosomal proteins L3 and L4"”one deletion (Q103del) and four substitutions (S113L, T35A, I98V, and N79D)"”were identified. Novel amino acid substitutions at positions E60K, G197D, and T285P of the OptrA protein were observed. MLST revealed 20 types of LRE strains; the most common type was ST16 (32.6%). PFGE showed 14 strains of ST16 with unique banding patterns. Eight novel sequence types (ST823 to ST830) and one allele (gki95) were identified for the first time in China.
CONCLUSIONS
optrA plays an important role in linezolid resistance and may serve as a marker for resistance screening. Since the L3 and L4 mutations did not simultaneously occur in the same strain, they play a negligible role in linezolid resistance. Epidemiological investigation suggested that the LRE cases were sporadic.
Keyword
MeSH Terms
Figure
Reference
-
1. Kloss P, Xiong L, Shinabarger DL, Mankin AS. Resistance mutations in 23 S rRNA identify the site of action of the protein synthesis inhibitor linezolid in the ribosomal peptidyl transferase center. J Mol Biol. 1999; 294:93–101. PMID: 10556031.2. Mendes RE, Deshpande LM, Jones RN. Linezolid update: stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms. Drug Resist Updat. 2014; 17:1–12. PMID: 24880801.3. Bonilla H, Huband MD, Seidel J, Schmidt H, Lescoe MK, McCurdy SP, et al. Multicity outbreak of linezolid-resistant Staphylococcus epidermidis associated with clonal spread of a cfr-containing strain. Clin Infect Dis. 2010; 51:796–800. PMID: 20726771.4. Wong A, Reddy SP, Smyth DS, Aguero-Rosenfeld ME, Sakoulas G, Robinson DA. Polyphyletic emergence of linezolid-resistant staphylococci in the United States. Antimicrob Agents Chemother. 2010; 54:742–748. PMID: 19933808.5. Chen H, Wu W, Ni M, Liu Y, Zhang J, Xia F, et al. Linezolid-resistant clinical isolates of enterococci and Staphylococcus cohnii from a multicentre study in China: molecular epidemiology and resistance mechanisms. Int J Antimicrob Agents. 2013; 42:317–321. PMID: 23880167.6. Diaz L, Kiratisin P, Mendes RE, Panesso D, Singh KV, Arias CA. Transferable plasmid-mediated resistance to linezolid due to cfr in a human clinical isolate of Enterococcus faecalis. Antimicrob Agents Chemother. 2012; 56:3917–3922. PMID: 22491691.7. Wang Y, Lv Y, Cai J, Schwarz S, Cui L, Hu Z, et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother. 2015; 70:2182–2190. PMID: 25977397.8. Cai J, Wang Y, Schwarz S, Zhang G, Chen S, Gu D, et al. High detection rate of the oxazolidinone resistance gene optrA in Enterococcus faecalis isolated from a Chinese anorectal surgery ward. Int J Antimicrob Agents. 2016; 48:757–759. PMID: 27751624.9. Sharkey LK, Edwards TA, O'Neill AJ. ABC-F proteins mediate antibiotic resistance through ribosomal protection. MBio. 2016; 7:e01975-15. PMID: 27006457.10. Jones RN, Ross JE, Bell JM, Utsuki U, Fumiaki I, Kobayashi I, et al. Zyvox Annual Appraisal of Potency and Spectrum program: linezolid surveillance program results for 2008. Diagn Microbiol Infect Dis. 2009; 65:404–413. PMID: 19913683.11. Ross JE, Farrell DJ, Mendes RE, Sader HS, Jones RN. Eight-year (2002-2009) summary of the linezolid (Zyvox® Annual Appraisal of Potency and Spectrum; ZAAPS) program in European countries. J Chemother. 2011; 23:71–76. PMID: 21571621.12. Patel SN, Memari N, Shahinas D, Toye B, Jamieson FB, Farrell DJ. Linezolid resistance in Enterococcus faecium isolated in Ontario, Canada. Diagn Microbiol Infect Dis. 2013; 77:350–353. PMID: 24095643.13. Cho SY, Kim HM, Chung DR, Kim SH, Huh HJ, Kang CI, et al. Resistance mechanisms and clinical characteristics of linezolid-resistant Enterococcus faecium isolates: a single-centre study in South Korea. J Glob Antimicrob Resist. 2018; 12:44–47. PMID: 28941790.14. Baquero F. Low-level antibacterial resistance: a gateway to clinical resistance. Drug Resist Updat. 2001; 4:93–105. PMID: 11512526.15. CLSI. Performance standards for antimicrobial susceptibility testing. M02-A12, M07-A10, and M11-A8. M100-table 2D. Wayne, PA: Clinical and Laboratory Standards Institute;2017.16. CLSI. Methods for dilution antimicrobial tests for bacteria that grow aerobically. Approved standard. 10th ed. CLSI M07-A10. 10th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2015.17. Bourgeois-Nicolaos N, Massias L, Couson B, Butel MJ, Andremont A, Doucet-Populaire F. Dose dependence of emergence of resistance to linezolid in Enterococcus faecalis in vivo. J Infect Dis. 2007; 195:1480–1488. PMID: 17436228.18. Wang L, He Y, Xia Y, Wang H, Liang S. Investigation of mechanism and molecular epidemiology of linezolid-resistant Enterococcus faecalis in China. Infect Genet Evol. 2014; 26:14–19. PMID: 24815727.19. Clewley JP, Arnold C. MEGALIGN. The multiple alignment module of Lasergene. Methods Mol Biol. 1997; 70:119–129. PMID: 9089607.20. Ruiz-Garbajosa P, Bonten MJ, Robinson DA, Top J, Nallapareddy SR, Torres C, et al. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J Clin Microbiol. 2006; 44:2220–2228. PMID: 16757624.21. Francisco AP, Vaz C, Monteiro PT, Melo-Cristino J, Ramirez M, Carriço JA. PHYLOViZ: phylogenetic inference and data visualization for sequence based typing methods. BMC Bioinformatics. 2012; 13:87. PMID: 22568821.22. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012; 18:268–281. PMID: 21793988.23. Liu Y, Wang Y, Schwarz S, Wang S, Chen L, Wu C, et al. Investigation of a multiresistance gene cfr that fails to mediate resistance to phenicols and oxazolidinones in Enterococcus faecalis. J Antimicrob Chemother. 2014; 69:892–898. PMID: 24272266.24. Li B, Ma CL, Yu X, Sun Y, Li MM, Ye JZ, et al. Investigation of mechanisms and molecular epidemiology of linezolid nonsusceptible Enterococcus faecalis isolated from a teaching hospital in China. J Microbiol Immunol Infect. 2016; 49:595–599. PMID: 26210761.25. Ikonomidis A, Grapsa A, Pavlioglou C, Demiri A, Batarli A, Panopoulou M. Accumulation of multiple mutations in linezolid-resistant Staphylococcus epidermidis causing bloodstream infections; in silico analysis of L3 amino acid substitutions that might confer high-level linezolid resistance. J Chemother. 2016; 28:465–468. PMID: 27077930.26. Locke JB, Hilgers M, Shaw KJ. Mutations in ribosomal protein L3 are associated with oxazolidinone resistance in staphylococci of clinical origin. Antimicrob Agents Chemother. 2009; 53:5275–5278. PMID: 19805557.27. Cai J, Wang Y, Schwarz S, Lv H, Li Y, Liao K, et al. Enterococcal isolates carrying the novel oxazolidinone resistance gene optrA from hospitals in Zhejiang, Guangdong, and Henan, China, 2010-2014. Clin Microbiol Infect. 2015; 21:1095.e1–1095.e4.28. Flamm RK, Mendes RE, Hogan PA, Streit JM, Ross JE, Jones RN. Linezolid surveillance results for the United States (LEADER Surveillance Program 2014). Antimicrob Agents Chemother. 2016; 60:2273–2280. PMID: 26833165.29. Cavaco LM, Bernal JF, Zankari E, Léon M, Hendriksen RS, Perez-Gutierrez E, et al. Detection of linezolid resistance due to the optrA gene in Enterococcus faecalis from poultry meat from the American continent (Colombia). J Antimicrob Chemother. 2017; 72:678–683. PMID: 27999039.30. Tamang MD, Moon DC, Kim SR, Kang HY, Lee K, Nam HM, et al. Detection of novel oxazolidinone and phenicol resistance gene optrA in enterococcal isolates from food animals and animal carcasses. Vet Microbiol. 2017; 201:252–256. PMID: 28284617.31. Hua RY, Xia Y, Wu WY, Yan J, Yang M. Whole transcriptome analysis reveals potential novel mechanisms of low-level linezolid resistance in Enterococcus faecalis. Gene. 2018; 647:143–149. PMID: 29325735.32. Cui L, Wang Y, Lv Y, Wang S, Song Y, Li Y, et al. Nationwide surveillance of novel oxazolidinone resistance gene optrA in Enterococcus isolates in China from 2004 to 2014. Antimicrob Agents Chemother. 2016; 60:7490–7493. PMID: 27645239.33. Li D, Wang Y, Schwarz S, Cai J, Fan R, Li J, et al. Co-location of the oxazolidinone resistance genes optrA and cfr on a multiresistance plasmid from Staphylococcus sciuri. J Antimicrob Chemother. 2016; 71:1474–1478. PMID: 26953332.34. Long KS, Vester B. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob Agents Chemother. 2012; 56:603–612. PMID: 22143525.35. Mendes RE, Deshpande LM, Farrell DJ, Spanu T, Fadda G, Jones RN. Assessment of linezolid resistance mechanisms among Staphylococcus epidermidis causing bacteraemia in Rome, Italy. J Antimicrob Chemother. 2010; 65:2329–2335. PMID: 20841419.36. Mendes RE, Flamm RK, Hogan PA, Ross JE, Jones RN. Summary of linezolid activity and resistance mechanisms detected during the 2012 LEADER surveillance program for the United States. Antimicrob Agents Chemother. 2014; 58:1243–1247. PMID: 24323470.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Emergence of optrA-Mediated Linezolid-Nonsusceptible Enterococcus faecalis in a Tertiary Care Hospital
- Susceptibility of Glycopeptide-Resistant Enterococci to Linezolid, Quinupristin/dalfopristin, Tigecycline and Daptomycin in a Tertiary Greek Hospital
- High Incidence of Virulence Factors Among Clinical Enterococcus faecalis Isolates in Southwestern Iran
- Epidemiology and Molecular Characterization of Vancomycin-Resistant Enterococcus faecalis
- Vancomycin-Resistant Enterococcus faecium Meningitis Treated with Linezolid: A Case Report and Review of the Literature