Ann Dermatol.
2018 Oct;30(5):581-587. 10.5021/ad.2018.30.5.581.
Platycodin D May Improve Acne and Prevent Scarring by Downregulating SREBP-1 Expression Via Inhibition of IGF-1R/PI3K/Akt Pathway and Modulating Inflammation with an Increase in Collagen
- Affiliations
-
- 1Laboratory of Cancer Immunology and Imaging, Seoul National University College of Medicine, Seoul, Korea.
- 2Acne, Rosacea, Seborrheic Dermatitis and Hidradenitis Suppurativa Research Laboratory, Seoul National University Hospital, Seoul, Korea.
- 3Department of Dermatology, University of Ulsan College of Medicine, Ulsan, Korea. yusungchoi9@gmail.com
- KMID: 2419749
- DOI: http://doi.org/10.5021/ad.2018.30.5.581
Abstract
- BACKGROUND
Although many therapeutic agents have been developed, only a few drugs are known to target multiple pathogenic factors in the treatment of acne.
OBJECTIVE
The purpose of this study was to identify a new drug candidate, platycodin D, which is a substance extracted from the root of Platycodon grandiflorum.
METHODS
Using western blotting and Cell Counting Kit-8 assay, we studied the effects of platycodin D on SEB-1 sebocytes, fibroblasts, and keratinocytes. We investigated its effects in view of lipogenesis, collagen production, anti-inflammatory activity, and dyskeratinization.
RESULTS
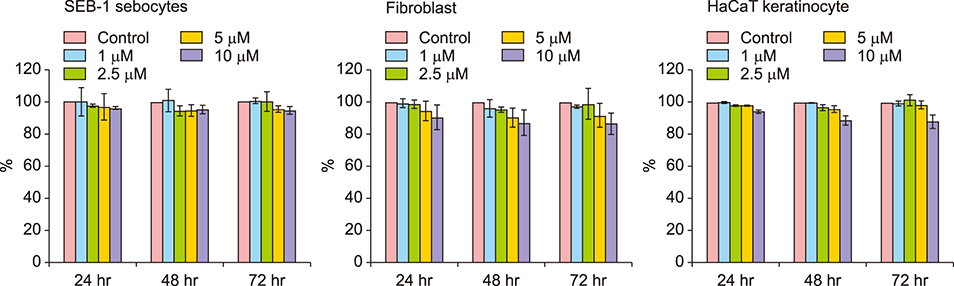
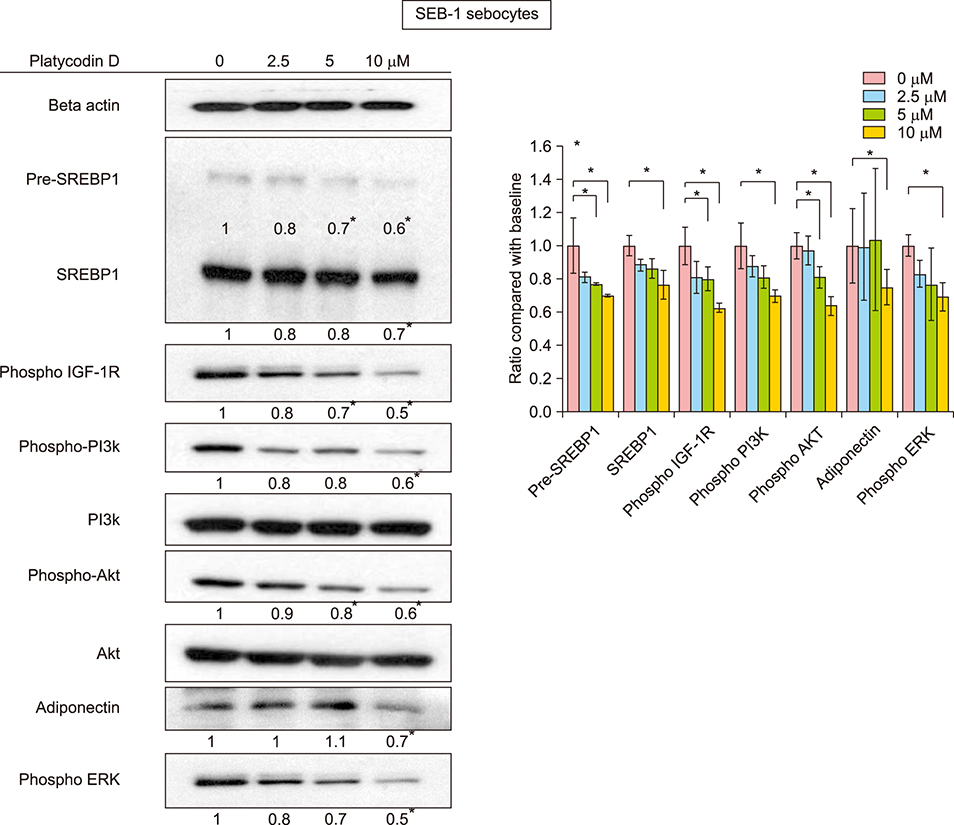
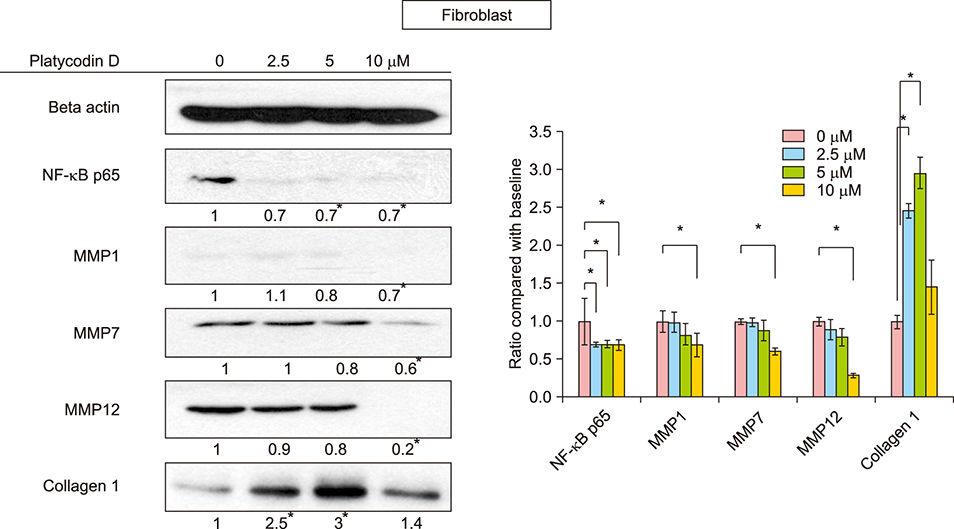
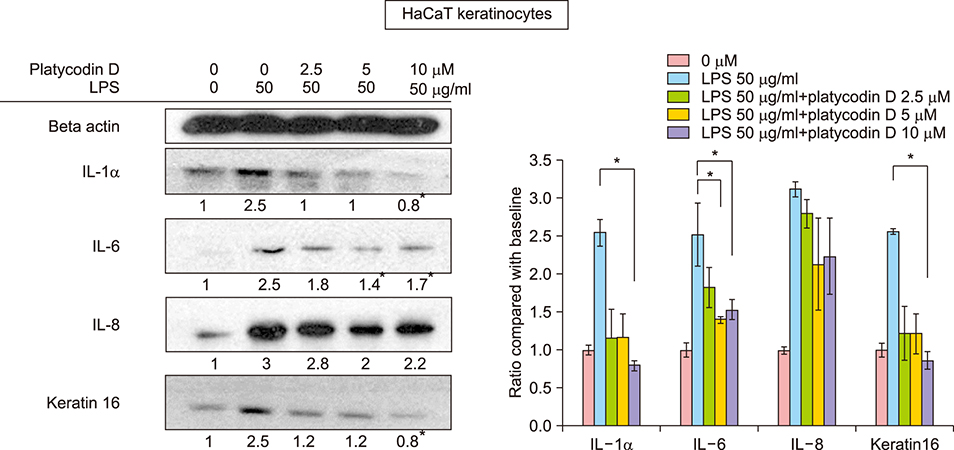
In SEB-1 sebocytes, platycodin D showed a sebosuppressive effect by downregulating ERK and insulin- like growth factor-1R/PI3K/Akt/sterol-regulatory element binding protein-1 signaling pathways. In addition, adiponectin, one of the adipokines responsible for sebum production, was decreased in platycodin D-treated SEB-1 sebocytes. In fibroblasts, platycodin D increased collagen production and reduced inflammation by inhibiting nuclear factor kappa B and matrix metalloproteinases. Platycodin D also showed anti-inflammatory effects on keratinocytes. It also suppressed keratin 16 expression induced by lipopolysaccharide. Furthermore, platycodin D showed no cytotoxicity on both SEB-1 sebocytes and fibroblasts.
CONCLUSION
Our data demonstrate the clinical feasibility of platycodin D for acne treatment and the prevention of acne scarring by sebosuppressive and anti-inflammatory effects, as well as through an increase in collagen levels.
Keyword
MeSH Terms
Figure
Reference
-
1. Li T, Chen X, Dai XY, Wei B, Weng QJ, Chen X, et al. Novel Hsp90 inhibitor platycodin D disrupts Hsp90/Cdc37 complex and enhances the anticancer effect of mTOR inhibitor. Toxicol Appl Pharmacol. 2017; 330:65–73.
Article2. Wang B, Gao Y, Zheng G, Ren X, Sun B, Zhu K, et al. Platycodin D inhibits interleukin-13-induced the expression of inflammatory cytokines and mucus in nasal epithelial cells. Biomed Pharmacother. 2016; 84:1108–1112.
Article3. Lee EJ, Kang M, Kim YS. Platycodin D inhibits lipogenesis through AMPKα-PPARγ2 in 3T3-L1 cells and modulates fat accumulation in obese mice. Planta Med. 2012; 78:1536–1542.
Article4. Xu C, Sun G, Yuan G, Wang R, Sun X. Effects of platycodin D on proliferation, apoptosis and PI3K/Akt signal pathway of human glioma U251 cells. Molecules. 2014; 19:21411–21423.
Article5. Qin H, Du X, Zhang Y, Wang R. Platycodin D, a triterpenoid saponin from platycodon grandiflorum, induces G2/M arrest and apoptosis in human hepatoma HepG2 cells by modulating the PI3K/Akt pathway. Tumour Biol. 2014; 35:1267–1274.
Article6. Thiboutot D, Jabara S, McAllister JM, Sivarajah A, Gilliland K, Cong Z, et al. Human skin is a steroidogenic tissue: steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1). J Invest Dermatol. 2003; 120:905–914.
Article7. Smith TM, Gilliland K, Clawson GA, Thiboutot D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008; 128:1286–1293.
Article8. Jung YR, Lee JH, Sohn KC, Lee Y, Seo YJ, Kim CD, et al. Adiponectin signaling regulates lipid production in human sebocytes. PLoS One. 2017; 12:e0169824.
Article9. Yoon JY, Kwon HH, Min SU, Thiboutot DM, Suh DH. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J Invest Dermatol. 2013; 133:429–440.
Article10. Kwon HH, Yoon JY, Park SY, Min S, Kim YI, Park JY, et al. Activity-guided purification identifies lupeol, a pentacyclic triterpene, as a therapeutic agent multiple pathogenic factors of acne. J Invest Dermatol. 2015; 135:1491–1500.
Article11. Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y, et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem. 1999; 274:35832–35839.
Article12. Lee WR, Kim KH, An HJ, Kim JY, Chang YC, Chung H, et al. The protective effects of melittin on propionibacterium acnes-induced inflammatory responses in vitro and in vivo. J Invest Dermatol. 2014; 134:1922–1930.
Article13. Kovács D, Lovászi M, Póliska S, Oláh A, Bíró T, Veres I, et al. Sebocytes differentially express and secrete adipokines. Exp Dermatol. 2016; 25:194–199.
Article14. Aydin K, Çetinözman F, Elcin G, Aksoy DY, Ucar F, Yildiz BO. Suppressed adiponectin levels and increased adiponectin response to oral glucose load in lean women with severe acne normalizes after isotretinoin treatment. Dermatology. 2017; 233:314–319.
Article15. Papakonstantinou E, Aletras AJ, Glass E, Tsogas P, Dionyssopoulos A, Adjaye J, et al. Matrix metalloproteinases of epithelial origin in facial sebum of patients with acne and their regulation by isotretinoin. J Invest Dermatol. 2005; 125:673–684.
Article16. Sato T, Shirane T, Noguchi N, Sasatsu M, Ito A. Novel anti-acne actions of nadifloxacin and clindamycin that inhibit the production of sebum, prostaglandin E(2) and promatrix metalloproteinase-2 in hamster sebocytes. J Dermatol. 2012; 39:774–780.
Article17. Jalian HR, Liu PT, Kanchanapoomi M, Phan JN, Legaspi AJ, Kim J. All-trans retinoic acid shifts propionibacterium acnes-induced matrix degradation expression profile toward matrix preservation in human monocytes. J Invest Dermatol. 2008; 128:2777–2782.
Article18. Choi JY, Piao MS, Lee JB, Oh JS, Kim IG, Lee SC. Propionibacterium acnes stimulates pro-matrix metalloproteinase-2 expression through tumor necrosis factor-alpha in human dermal fibroblasts. J Invest Dermatol. 2008; 128:846–854.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Curcumin targets vascular endothelial growth factor viaactivating the PI3K/Akt signaling pathway and improves brainhypoxic-ischemic injury in neonatal rats
- IGF-I Exerts an Anti-inflammatory Effect on Skeletal Muscle Cells through Down-regulation of TLR4 Signaling
- Klotho plays a critical role in clear cell renal cell carcinoma progression and clinical outcome
- Isorhamnetin Alleviates Inflammation-Induced Crosstalk between Kynurenine Pathway and Gut Microbiota in Depressed Mice
- Inflammatory cytokines in midbrain periaqueductal gray contribute to diabetic induced pain hypersensitivity through phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway