Intest Res.
2018 Jul;16(3):384-392. 10.5217/ir.2018.16.3.384.
β-(1,3)-Glucan derived from Candida albicans induces inflammatory cytokines from macrophages and lamina propria mononuclear cells derived from patients with Crohn's disease
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan. nagamakoto@z7.keio.jp takagast@keio.jp
- 2Department of Surgery, Yokohama Municipal Citizen's Hospital, Yokohama, Japan.
- 3The Third Department of Internal Medicine, Kyorin University School of Medicine, Tokyo, Japan.
- KMID: 2417650
- DOI: http://doi.org/10.5217/ir.2018.16.3.384
Abstract
- BACKGROUND/AIMS
Recent research has highlighted the importance of interactions between commensal fungi and intestinal inflammation. However, there are few studies investigating whether commensal fungi contribute to inflammation in patients with Crohn's disease (CD). The aim of this study is to investigate reveal interactions between commensal fungi and host immune cells in CD.
METHODS
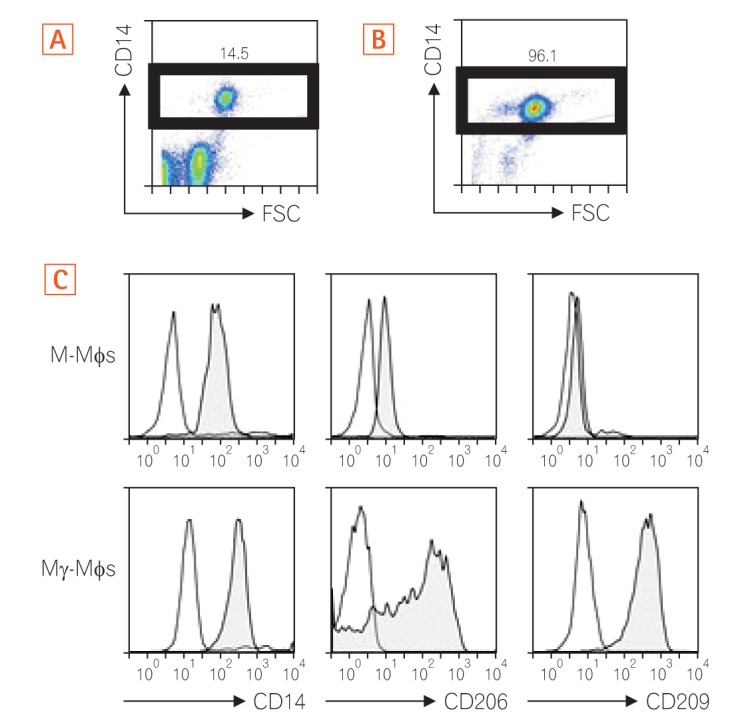
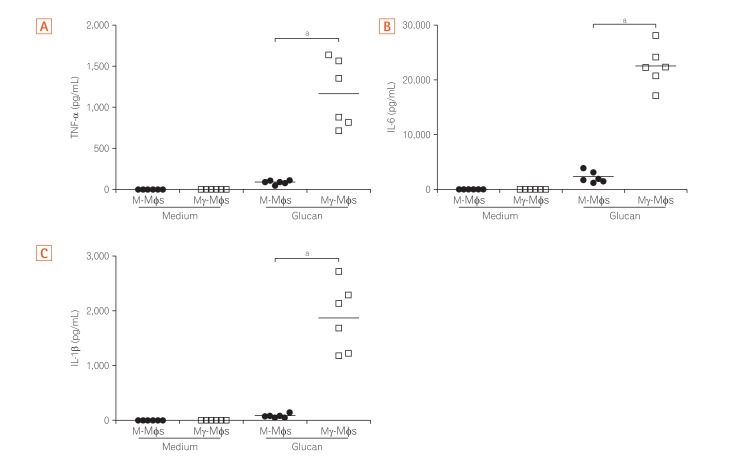
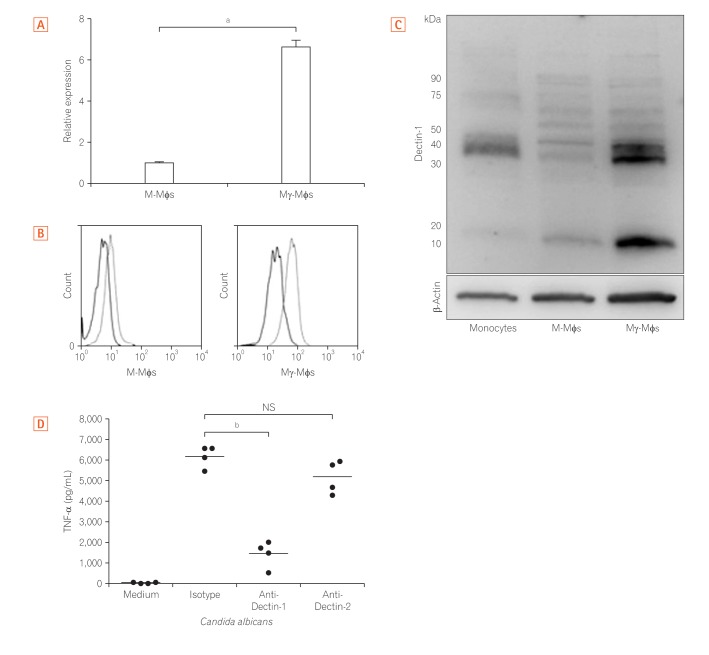
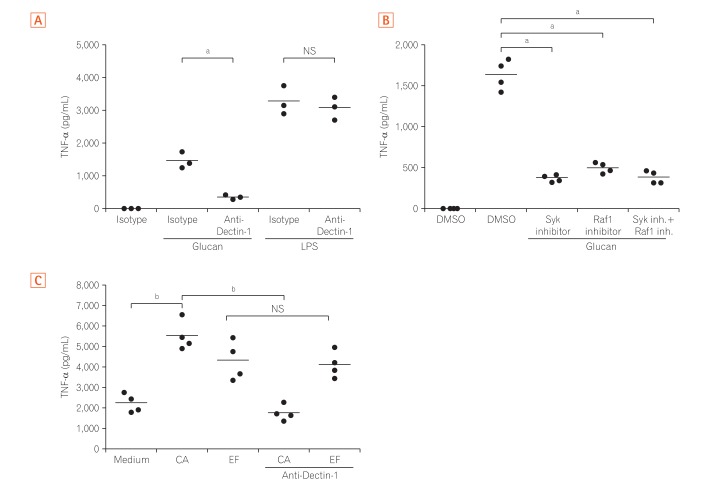
CD14-positive monocytes were isolated from peripheral blood mononuclear cells from healthy human volunteers and then differentiated in the presence of macrophage colony-stimulating factor (M-CSF) (referred to as M-macrophages, M-Mϕs) or M-CSF and interferon-γ (IFN-γ) (referred to as M-gamma macrophages, Mγ-Mϕs). Cytokine production by these in vitro differentiated macrophages in response to β-(1,3)-glucan was analyzed by flow cytometry. Expression of Dectin-1 was examined using flow cytometry, western blotting, and quantitative reverse transcription-polymerase chain reaction. Cytokine production by in vitro differentiated macrophages in response to β-(1,3)-glucan was measured in the presence of an anti-Dectin-1 receptor antagonist, anti-Syr, or an anti-Fas-1 antibody. Cytokine production by lamina propria mononuclear cells (LPMCs) derived from CD patients in response to β-(1,3)-glucan was also analyzed.
RESULTS
Mγ-Mϕs produced a large amount of tumor necrosis factor-α (TNF-α) and interleukin-6 in response to β-(1,3)-glucan. Dectin-1 expression was significantly higher in Mγ-Mϕs than in M-Mϕs. The increase in TNF-α production by Mγ-Mϕs stimulated with glucan was reversed by blocking Dectin-1, Syr or Fas-1. LPMCs derived from CD patients stimulated with β-(1,3)-glucan produced significantly higher amount of TNF-α than LPMCs derived from UC patients.
CONCLUSIONS
These results suggest that commensal fungal microbiota may contribute to the pathogenesis of CD by inducing macrophages-derived pro-inflammatory cytokines.
MeSH Terms
-
Blotting, Western
Candida albicans*
Candida*
Crohn Disease*
Cytokines*
Flow Cytometry
Fungi
Healthy Volunteers
Humans
In Vitro Techniques
Inflammation
Interleukin-6
Macrophage Colony-Stimulating Factor
Macrophages*
Microbiota
Monocytes
Mucous Membrane*
Necrosis
Tumor Necrosis Factor-alpha
Cytokines
Interleukin-6
Macrophage Colony-Stimulating Factor
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Naganuma M, Sakuraba A, Hibi T. Ulcerative colitis: prevention of relapse. Expert Rev Gastroenterol Hepatol. 2013; 7:341–351. PMID: 23639092.
Article2. Yun L, Hanauer S. Selecting appropriate anti-TNF agents in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2009; 3:235–248. PMID: 19485806.
Article3. Lewis JD, Chen EZ, Baldassano RN, et al. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn's disease. Cell Host Microbe. 2015; 18:489–500. PMID: 26468751.4. Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006; 3:390–407. PMID: 16819502.
Article5. Sartor RB. Therapeutic correction of bacterial dysbiosis discovered by molecular techniques. Proc Natl Acad Sci U S A. 2008; 105:16413–16414. PMID: 18948599.
Article6. Kamada N, Hisamatsu T, Honda H, et al. Human CD14+ macrophages in intestinal lamina propria exhibit potent antigen-presenting ability. J Immunol. 2009; 183:1724–1731. PMID: 19592647.7. Kamada N, Hisamatsu T, Okamoto S, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008; 118:2269–2280. PMID: 18497880.8. Yoneno K, Hisamatsu T, Shimamura K, et al. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn's disease. Immunology. 2013; 139:19–29. PMID: 23566200.
Article9. Sendid B, Colombel JF, Jacquinot PM, et al. Specific antibody response to oligomannosidic epitopes in Crohn's disease. Clin Diagn Lab Immunol. 1996; 3:219–226. PMID: 8991640.10. Standaert-Vitse A, Jouault T, Vandewalle P, et al. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn's disease. Gastroenterology. 2006; 130:1764–1775. PMID: 16697740.11. Chehoud C, Albenberg LG, Judge C, et al. Fungal signature in the gut microbiota of pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015; 21:1948–1956. PMID: 26083617.12. Kühbacher T, Ott SJ, Helwig U, et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006; 55:833–841. PMID: 16401690.13. Kumamoto CA. Inflammation and gastrointestinal Candida colonization. Curr Opin Microbiol. 2011; 14:386–391. PMID: 21802979.14. Li Q, Wang C, Tang C, He Q, Li N, Li J. Dysbiosis of gut fungal microbiota is associated with mucosal inflammation in Crohn's disease. J Clin Gastroenterol. 2014; 48:513–523. PMID: 24275714.
Article15. Liguori G, Lamas B, Richard ML, et al. Fungal dysbiosis in mucosa-associated microbiota of Crohn's disease patients. J Crohns Colitis. 2016; 10:296–305. PMID: 26574491.
Article16. Mukhopadhya I, Hansen R, Meharg C, et al. The fungal microbiota of de-novo paediatric inflammatory bowel disease. Microbes Infect. 2015; 17:304–310. PMID: 25522934.
Article17. Sokol H, Leducq V, Aschard H, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017; 66:1039–1048. PMID: 26843508.
Article18. Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr Opin Immunol. 2015; 32:21–27. PMID: 25553393.
Article19. Rizzetto L, De Filippo C, Rivero D, Riccadonna S, Beltrame L, Cavalieri D. Systems biology of host-mycobiota interactions: dissecting Dectin-1 and Dectin-2 signalling in immune cells with DC-ATLAS. Immunobiology. 2013; 218:1428–1437. PMID: 23932568.
Article20. Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003; 197:1119–1124. PMID: 12719478.21. Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009; 9:465–479. PMID: 19521399.
Article22. Zwolinska-Wcislo M, Brzozowski T, Budak A, et al. Effect of Candida colonization on human ulcerative colitis and the healing of inflammatory changes of the colon in the experimental model of colitis ulcerosa. J Physiol Pharmacol. 2009; 60:107–118.23. Jawhara S, Thuru X, Standaert-Vitse A, et al. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J Infect Dis. 2008; 197:972–980. PMID: 18419533.
Article24. Suzuki H, Hisamatsu T, Chiba S, et al. Glycolytic pathway affects differentiation of human monocytes to regulatory macrophages. Immunol Lett. 2016; 176:18–27. PMID: 27208804.
Article25. Saijo S, Fujikado N, Furuta T, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007; 8:39–46. PMID: 17159982.
Article26. Saijo S, Ikeda S, Yamabe K, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010; 32:681–691. PMID: 20493731.
Article27. Gow NA, Netea MG, Munro CA, et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis. 2007; 196:1565–1571. PMID: 18008237.
Article28. Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013; 500:232–236. PMID: 23842501.
Article29. Hayashi A, Sato T, Kamada N, et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013; 13:711–722. PMID: 23768495.
Article30. Kashiwagi I, Morita R, Schichita T, et al. Smad2 and Smad3 inversely regulate TGF-beta autoinduction in Clostridium butyricum-activated dendritic cells. Immunity. 2015; 43:65–79. PMID: 26141582.
Article31. Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998; 66:5224–5231. PMID: 9784526.
Article32. Iliev ID, Funari VA, Taylor KD, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012; 336:1314–1317. PMID: 22674328.
Article33. Brown GD, Gordon S. Immune recognition: a new receptor for beta-glucans. Nature. 2001; 413:36–37.34. Gringhuis SI, den Dunnen J, Litjens M, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol. 2009; 10:203–213. PMID: 19122653.
Article35. LeibundGut-Landmann S, Gross O, Robinson MJ, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007; 8:630–638. PMID: 17450144.
Article36. Skrzypek F, Cenci E, Pietrella D, Rachini A, Bistoni F, Vecchiarelli A. Dectin-1 is required for human dendritic cells to initiate immune response to Candida albicans through Syk activation. Microbes Infect. 2009; 11:661–670. PMID: 19358895.
Article37. Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009; 206:299–311. PMID: 19204111.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Candida spp.- Induced Cytokine Gene Expression on Mouse Peritoneal Macrophages and NIH 3T3 Fibroblasts
- IL-33 Priming Enhances Peritoneal Macrophage Activity in Response to Candida albicans
- Immunomodulatory effect of beta-glucan derived from Saccharomyces cerevisiae strains
- Regulation of Macrophage Activation and Differentiation in Atherosclerosis
- Immunological Abnormalities in the Pathogenesis of Inflammatory Bowel Disease