J Vet Sci.
2018 Jul;19(4):477-482. 10.4142/jvs.2018.19.4.477.
Porcine intestinal lymphoid tissues synthesize estradiol
- Affiliations
-
- 1Department of Comparative Biosciences, College of Veterinary Medicine, University of Illinois, Urbana-Campaign, IL 61802, USA. jayko@illinois.edu
- 2Department of Agricultural Biotechnology, Seoul National University, Seoul 08826, Korea.
- 3Department of Toxicology, Faculty of Veterinary Medicine, Benha University, Benha 13518, Egypt.
- KMID: 2417561
- DOI: http://doi.org/10.4142/jvs.2018.19.4.477
Abstract
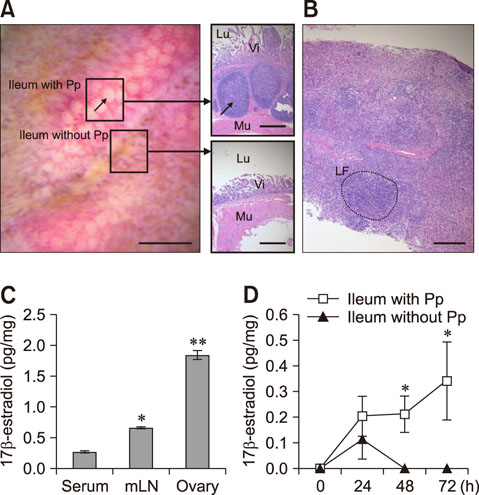
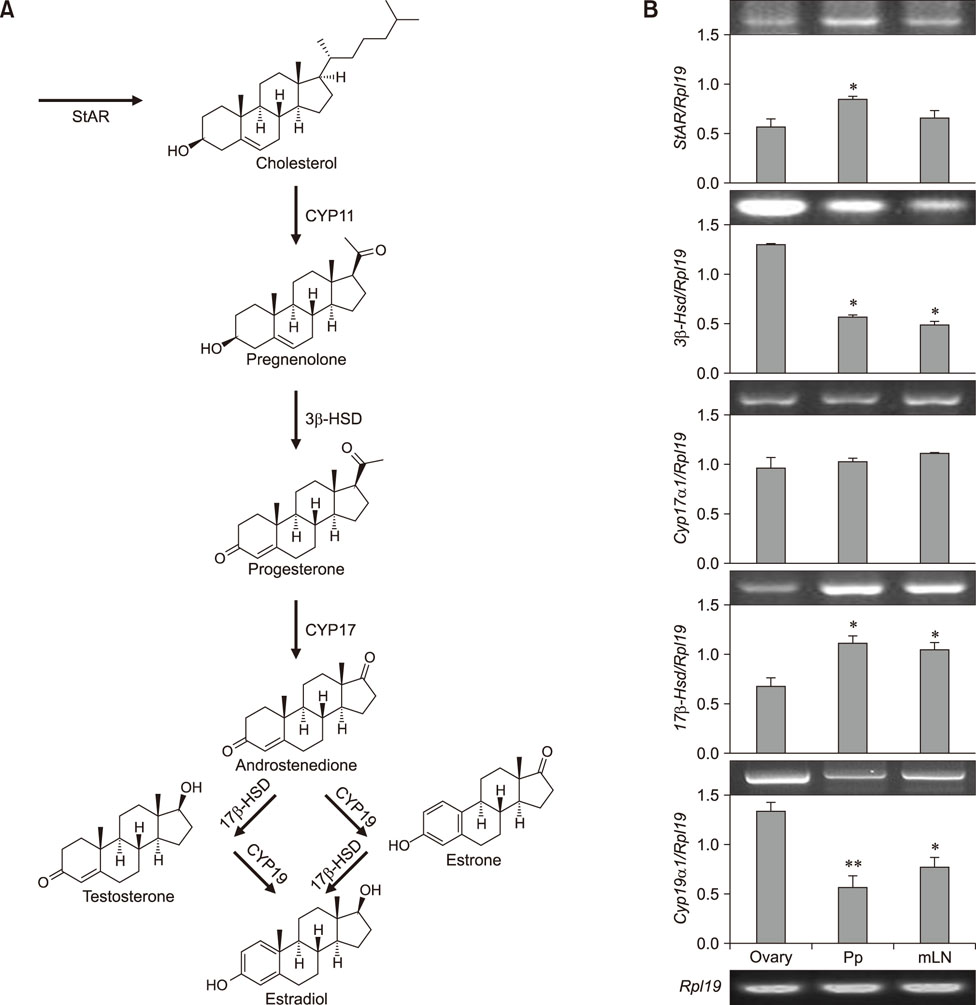
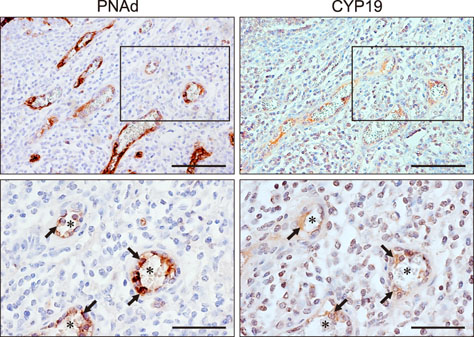
- Estradiol (17β-estradiol) is synthesized primarily in the gonads of both sexes and regulates the development and function of reproductive organs. Recently, we reported that intestinal lymphocyte homeostasis is regulated by estradiol synthesized de novo in the endothelial cells of the high endothelial venules (HEVs) of mesenteric lymph nodes and Peyer's patches in mice. This observation prompted us to hypothesize that HEVs of intestinal lymphoid tissues are sites of estradiol synthesis across species. In this study, we examined whether estradiol is synthesized in the intestinal lymphoid tissues of adolescent piglets. Comparisons of estradiol levels in blood and tissue showed that estradiol concentrations in mesenteric lymph nodes and Peyer's patches were significantly higher than the level in serum. Reverse transcription polymerase chain reaction showed that porcine intestinal lymphoid tissues express mRNAs for steroidogenic enzymes (StAR, 17β-Hsd, 3β-Hsd, Cyp17a1, and Cyp19a1), and immunohistochemical results in ilial tissue showed expression of aromatase (CYP19) in Peyer's patch-localized endothelial cells of HEVs. When mesenteric lymph node and Peyer's patch tissues were cultured in vitro, they produced estradiol. Taken together, the results indicate that mesenteric lymph nodes and Peyer's patches are sites of estradiol synthesis in adolescent piglets.
Keyword
MeSH Terms
Figure
Reference
-
1. Barakat R, Oakley O, Kim H, Jin J, Ko CJ. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016; 49:488–496.
Article2. Bazer FW, Thatcher WW, Martinat-Botte F, Terqui M. Sexual maturation and morphological development of the reproductive tract in Large White and prolific Chinese Meishan pigs. J Reprod Fertil. 1988; 83:723–728.
Article3. Berkovitz GD, Brown TR, Fujimoto M. Aromatase activity in human skin fibroblasts grown in cell culture. Steroids. 1987; 50:281–295.
Article4. Chen KL, Madak-Erdogan Z. Estrogen and microbiota crosstalk: should we pay attention? Trends Endocrinol Metab. 2016; 27:752–755.
Article5. Cleland WH, Mendelson CR, Simpson ER. Aromatase activity of membrane fractions of human adipose tissue stromal cells and adipocytes. Endocrinology. 1983; 113:2155–2160.
Article6. Colciago A, Celotti F, Pravettoni A, Mornati O, Martini L, Negri-Cesi P. Dimorphic expression of testosterone metabolizing enzymes in the hypothalamic area of developing rats. Brain Res Dev Brain Res. 2005; 155:107–116.
Article7. Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 2013; 19:197–209.
Article8. D'Errico I, Moschetta A. Nuclear receptors, intestinal architecture and colon cancer: an intriguing link. Cell Mol Life Sci. 2008; 65:1523–1543.9. Eberl G, Lochner M. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2009; 2:478–485.
Article10. Gomez A, Luckey D, Taneja V. The gut microbiome in autoimmunity: sex matters. Clin Immunol. 2015; 159:154–162.
Article11. Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y. Localized expression of aromatase in human vascular tissues. Circ Res. 1999; 84:1285–1291.
Article12. Hata S, Miki Y, Saito R, Ishida K, Watanabe M, Sasano H. Aromatase in human liver and its diseases. Cancer Med. 2013; 2:305–315.
Article13. Jung C, Hugot JP, Barreau F. Peyer's patches: the immune sensors of the intestine. Int J Inflam. 2010; 2010:823710.
Article14. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015; 294:63–69.
Article15. Lan YL, Zhao J, Li S. Update on the neuroprotective effect of estrogen receptor alpha against Alzheimer's disease. J Alzheimers Dis. 2015; 43:1137–1148.
Article16. Lélu K, Laffont S, Delpy L, Paulet PE, Périnat T, Tschanz SA, Pelletier L, Engelhardt B, Guéry JC. Estrogen receptor XMLLink_XYZ signaling in T lymphocytes is required for estradiol- mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol. 2011; 187:2386–2393.
Article17. Lin S, Ji W. Association between insulin resistance and estrogen in sexual precocity of obese children. Exp Ther Med. 2016; 12:2497–2500.
Article18. Lucky AW, Henderson TA, Olson WH, Robisch DM, Lebwohl M, Swinyer LJ. Effectiveness of norgestimate and ethinyl estradiol in treating moderate acne vulgaris. J Am Acad Dermatol. 1997; 37:746–754.
Article19. Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013; 339:1084–1088.
Article20. McMurray RW. Estrogen, prolactin, and autoimmunity: actions and interactions. Int Immunopharmacol. 2001; 1:995–1008.
Article21. Mela V, Vargas A, Meza C, Kachani M, Wagner EJ. Modulatory influences of estradiol and other anorexigenic hormones on metabotropic, Gi/o-coupled receptor function in the hypothalamic control of energy homeostasis. J Steroid Biochem Mol Biol. 2016; 160:15–26.
Article22. Moravek MB, Yin P, Ono M, Coon JS V, Dyson MT, Navarro A, Marsh EE, Chakravarti D, Kim JJ, Wei JJ, Bulun SE. Ovarian steroids, stem cells and uterine leiomyoma: therapeutic implications. Hum Reprod Update. 2015; 21:1–12.
Article23. Mulak A, Taché Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol. 2014; 20:2433–2448.
Article24. Oakley OR, Kim KJ, Lin PC, Barakat R, Cacioppo JA, Li Z, Whitaker A, Chung KC, Mei W, Ko C. Estradiol synthesis in gut-associated lymphoid tissue: leukocyte regulation by a sexually monomorphic system. Endocrinology. 2016; 157:4579–4587.
Article25. Orczyk GP, Behrman HR. Ovulation blockade by aspirin or indomethacin--in vivo evidence for a role of prostaglandin in gonadotrophin secretion. Prostaglandins. 1972; 1:3–20.
Article26. Oxender WD, Colenbrander B, van deWiel DF, Wensing CJ. Ovarian development in fetal and prepubertal pigs. Biol Reprod. 1979; 21:715–721.
Article27. Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol. 1994; 140:256–261.
Article28. Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005; 97:107–113.
Article29. Polan ML, Totora M, Caldwell BV, DeCherney AH, Haseltine FP, Kase N. Abnormal ovarian cycles as diagnosed by ultrasound and serum estradiol levels. Fertil Steril. 1982; 37:342–347.
Article30. Priyanka HP, Krishnan HC, Singh RV, Hima L, Thyagarajan S. Estrogen modulates in vitro T cell responses in a concentration- and receptor-dependent manner: effects on intracellular molecular targets and antioxidant enzymes. Mol Immunol. 2013; 56:328–339.
Article31. Richelson LS, Wahner HW, Melton LJ 3rd, Riggs BL. Relative contributions of aging and estrogen deficiency to postmenopausal bone loss. N Engl J Med. 1984; 311:1273–1275.
Article32. Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014; 345:771–776.
Article33. Samy TS, Knöferl MW, Zheng R, Schwacha MG, Bland KI, Chaudry IH. Divergent immune responses in male and female mice after trauma-hemorrhage: dimorphic alterations in T lymphocyte steroidogenic enzyme activities. Endocrinology. 2001; 142:3519–3529.
Article34. Sasano H, Uzuki M, Sawai T, Nagura H, Matsunaga G, Kashimoto O, Harada N. Aromatase in human bone tissue. J Bone Miner Res. 1997; 12:1416–1423.
Article35. Schreihofer DA, Ma Y. Estrogen receptors and ischemic neuroprotection: who, what, where, and when? Brain Res. 2013; 1514:107–122.
Article36. Wada-Hiraike O, Imamov O, Hiraike H, Hultenby K, Schwend T, Omoto Y, Warner M, Gustafsson JA. Role of estrogen receptor XMLLink_XYZ in colonic epithelium. Proc Natl Acad Sci U S A. 2006; 103:2959–2964.37. Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006; 116:1186–1194.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distribution and characterization of IL-10-secreting cells in lymphoid tissues of PCV2-infected pigs
- Quantification and genotyping of PCV2 DNA in the tissues of PCV2-infected conventional pigs with different clinical signs
- Effect of 17beta-estradiol on the Contraction to Endothelin-1 in Porcine Coronary Artery
- Roles of Embryonic and Adult Lymphoid Tissue Inducer Cells in Primary and Secondary Lymphoid Tissues
- A study of serum estradiol level and ultrasonographic follicular response in controlled hyperstimulated cycles of one or two ovaries