J Vet Sci.
2010 Sep;11(3):177-183. 10.4142/jvs.2010.11.3.177.
Distribution and characterization of IL-10-secreting cells in lymphoid tissues of PCV2-infected pigs
- Affiliations
-
- 1School of Veterinary Medicine and Biomedical Sciences, University of Nebraska-Lincoln, Lincoln, Nebraska 68583, USA.
- 2School of Veterinary and Biomedical Sciences and Nebraska Center for Virology, University of Nebraska-Lincoln, Lincoln, Nebraska 68583, USA.
- 3Department of Veterinary Pathology, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea. jsur@konkuk.ac.kr
- KMID: 1093490
- DOI: http://doi.org/10.4142/jvs.2010.11.3.177
Abstract
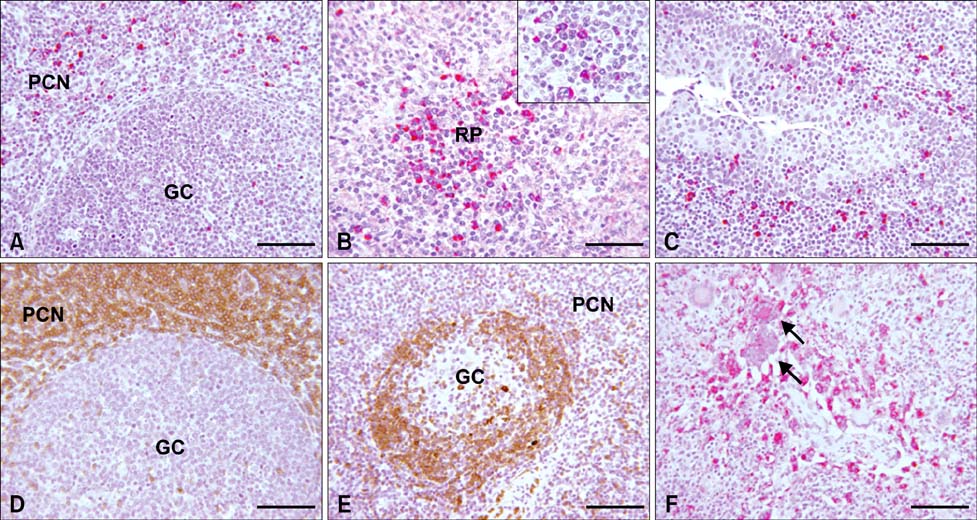
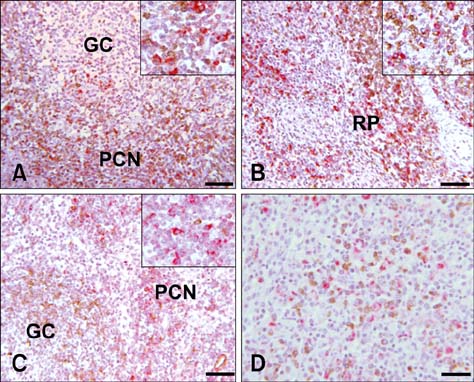
- Distribution and characterization of interlukin-10 (IL-10)-secreting cells in lymphoid tissues of pigs naturally infected with porcine circovirus type 2 (PCV2) were evaluated in accordance with PCV2 antigen detection. After screening a total of 56 pigs showing the symptoms of postweaning multisystemic wasting syndrome (PMWS), 15 pigs were PCV2 positive and 5 pigs, which showed stronger positive signals over multiples tissues were further investigated. This study showed that in PCV2-infected lymphoid tissues, particularly mandibular lymph node, spleen and tonsil, IL-10 expression was mainly localized in T-cell rich areas but rarely in B cell rich areas. IL-10 was highly expressed in bystander cells but rarely in PCV2-infected cells. Elevated IL-10 expression was predominantly associated with T cells, but rarely with B cells or with macrophages. The results of this study provide evidence for the role of IL-10 in chronic PCV2 infection and its relation to PCV2 antigen in affected tissues. Constantly elevated levels of IL-10 lead to immunosuppression in persistent and chronic viral infections. The increased IL-10 expression observed in PCV2 infection in this study suggests that IL-10-mediated immunosuppression may play an important role in the pathogenesis and maintenance of naturally occurring PCV2 infection.
MeSH Terms
-
Animals
Circoviridae Infections/immunology/pathology/*veterinary
Circovirus/*immunology
Gene Expression Regulation/*immunology
Immunohistochemistry/veterinary
Interleukin-10/immunology/*secretion
Lymphoid Tissue/immunology/*pathology/secretion
Porcine Postweaning Multisystemic Wasting Syndrome/*immunology/pathology
Republic of Korea
Swine
T-Lymphocytes/immunology
Figure
Reference
-
1. Allan GM, McNeilly F, Kennedy S, Daft B, Clarke EG, Ellis JA, Haines DM, Meehan BM, Adair BM. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J Vet Diagn Invest. 1998. 10:3–10.
Article2. Barrett L, Gallant M, Howley C, Bowmer MI, Hirsch G, Peltekian K, Grant M. Enhanced IL-10 production in response to hepatitis C virus proteins by peripheral blood mononuclear cells from human immunodeficiency virus-monoinfected individuals. BMC Immunol. 2008. 9:28.
Article3. Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006. 12:1301–1309.
Article4. Chae C. Postweaning multisystemic wasting syndrome: a review of aetiology, diagnosis and pathology. Vet J. 2004. 168:41–49.
Article5. Chang HW, Jeng CR, Lin CM, Liu JJ, Chang CC, Tsai YC, Chia MY, Pang VF. The involvement of Fas/FasL interaction in porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus co-inoculation-associated lymphocyte apoptosis in vitro. Vet Microbiol. 2007. 122:72–82.
Article6. Chung HK, Chae C. Expression of interleukin-10 and interleukin-12 in piglets experimentally infected with porcine reproductive and respiratory syndrome virus (PRRSV). J Comp Pathol. 2003. 129:205–212.
Article7. Darwich L, Balasch M, Plana-Durán J, Segalés J, Domingo M, Mateu E. Cytokine profiles of peripheral blood mononuclear cells from pigs with postweaning multisystemic wasting syndrome in response to mitogen, superantigen or recall viral antigens. J Gen Virol. 2003. 84:3453–3457.
Article8. Darwich L, Pié S, Rovira A, Segalés J, Domingo M, Oswald IP, Mateu E. Cytokine mRNA expression profiles in lymphoid tissues of pigs naturally affected by postweaning multisystemic wasting syndrome. J Gen Virol. 2003. 84:2117–2125.
Article9. Darwich L, Segalés J, Resendes A, Balasch M, Plana-Durán J, Mateu E. Transient correlation between viremia levels and IL-10 expression in pigs subclinically infected with porcine circovirus type 2 (PCV2). Res Vet Sci. 2008. 84:194–198.
Article10. Faldyna M, Samankova P, Leva L, Cerny J, Oujezdska J, Rehakova Z, Sinkora J. Cross-reactive anti-human monoclonal antibodies as a tool for B-cell identification in dogs and pigs. Vet Immunol Immunopathol. 2007. 119:56–62.
Article11. Gómez-Laguna J, Salguero FJ, Barranco I, Pallarés FJ, Rodríguez-Gómez IM, Bernabé A, Carrasco L. Cytokine expression by macrophages in the lung of pigs infected with the porcine reproductive and respiratory syndrome virus. J Comp Pathol. 2010. 142:51–60.
Article12. Grierson SS, King DP, Tucker AW, Donadeu M, Mellencamp MA, Haverson K, Banks M, Bailey M. Ontogeny of systemic cellular immunity in the neonatal pig: correlation with the development of post-weaning multisystemic wasting syndrome. Vet Immunol Immunopathol. 2007. 119:254–268.
Article13. Kekarainen T, Montoya M, Mateu E, Segalés J. Porcine circovirus type 2-induced interleukin-10 modulates recall antigen responses. J Gen Virol. 2008. 89:760–765.
Article14. Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993. 11:165–190.
Article15. Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008. 226:205–218.
Article16. Opriessnig T, Meng XJ, Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007. 19:591–615.
Article17. Quintana J, Segalés J, Rosell C, Calsamiglia M, Rodríguez-Arrioja GM, Chianini F, Folch JM, Maldonado J, Canal M, Plana-Durán J, Domingo M. Clinical and pathological observations on pigs with postweaning multisystemic wasting syndrome. Vet Rec. 2001. 149:357–361.
Article18. Rosell C, Segalés J, Plana-Durán J, Balasch M, Rodríguez-Arrioja GM, Kennedy S, Allan GM, McNeilly F, Latimer KS, Domingo M. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J Comp Pathol. 1999. 120:59–78.
Article19. Segalés J, Domingo M, Chianini F, Majó N, Domínguez J, Darwich L, Mateu E. Immunosuppression in postweaning multisystemic wasting syndrome affected pigs. Vet Microbiol. 2004. 98:151–158.
Article20. Segalés J, Rosell C, Domingo M. Pathological findings associated with naturally acquired porcine circovirus type 2 associated disease. Vet Microbiol. 2004. 98:137–149.
Article21. Sipos W, Duvigneau JC, Willheim M, Schilcher F, Hartl RT, Hofbauer G, Exel B, Pietschmann P, Schmoll F. Systemic cytokine profile in feeder pigs suffering from natural postweaning multisystemic wasting syndrome (PMWS) as determined by semiquantitative RT-PCR and flow cytometric intracellular cytokine detection. Vet Immunol Immunopathol. 2004. 99:63–71.
Article22. Stevenson LS, McCullough K, Vincent I, Gilpin DF, Summerfield A, Nielsen J, McNeilly F, Adair BM, Allan GM. Cytokine and C-reactive protein profiles induced by porcine circovirus type 2 experimental infection in 3-week-old piglets. Viral Immunol. 2006. 19:189–195.
Article23. Tischer I, Gelderblom H, Vettermann W, Koch MA. A very small porcine virus with circular single-stranded DNA. Nature. 1982. 295:64–66.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quantification and genotyping of PCV2 DNA in the tissues of PCV2-infected conventional pigs with different clinical signs
- Pathologic studies in lymph nodes of pigs infected with porcine circovirus type 2, porcine reproductive and respiratory syndrome virus
- Distribution of Interleukin-22–secreting Immune Cells in Conjunctival Associated Lymphoid Tissue
- Heterogeneity of IL-22-producing Lymphoid Tissue Inducer-like Cells in Human and Mouse
- Porcine circovirus type 2 increases interleukin-1beta and interleukin-10 production via the MyD88–NF-kappa B signaling pathway in porcine alveolar macrophages in vitro