J Vet Sci.
2017 Jun;18(2):183-191. 10.4142/jvs.2017.18.2.183.
Porcine circovirus type 2 increases interleukin-1beta and interleukin-10 production via the MyD88–NF-kappa B signaling pathway in porcine alveolar macrophages in vitro
- Affiliations
-
- 1College of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, China. lyj@njau.edu.cn
- KMID: 2412571
- DOI: http://doi.org/10.4142/jvs.2017.18.2.183
Abstract
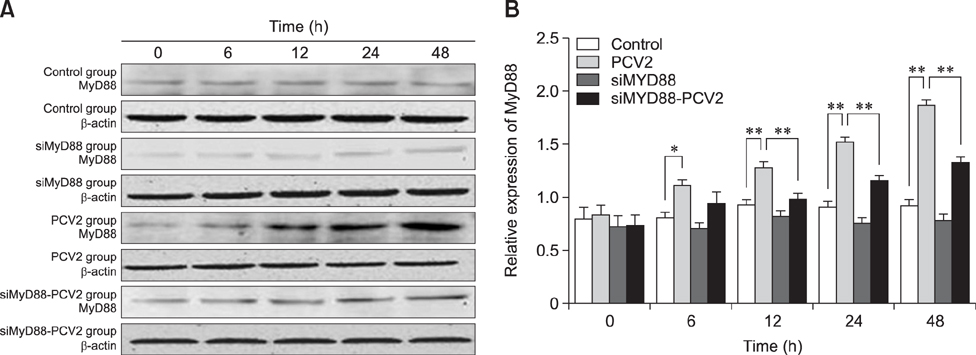
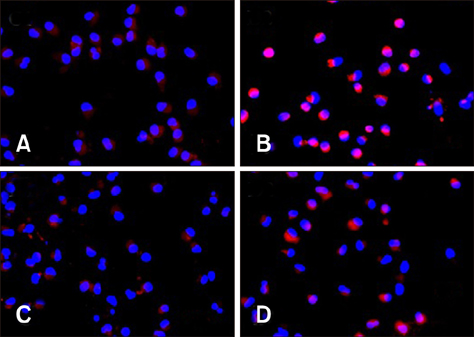
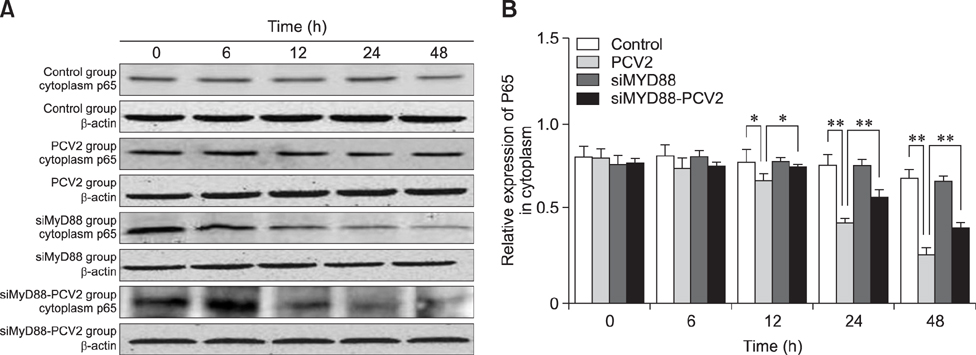
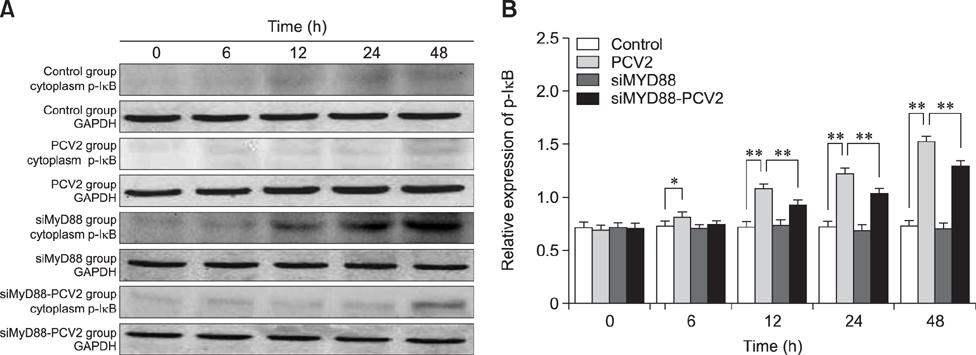
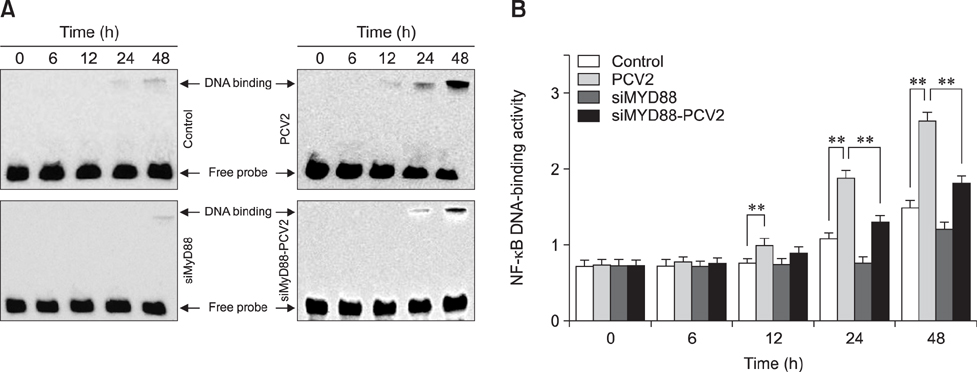
- Porcine alveolar macrophages (PAMs) represent the first line of defense in the porcine lung after infection with porcine circovirus type 2 (PCV2) via the respiratory tract. However, PCV2 infection impairs the microbicidal capability of PAMs and alters cytokine production and/or secretion. At present, the reason for the imbalance of cytokines has not been fully elucidated, and the regulatory mechanisms involved are unclear. In this study, we investigated the expression levels and regulation of interleukin-1beta (IL-1β) and IL-10 in PAMs following incubation with PCV2 in vitro. Levels of IL-1β and IL-10 increased in PAM supernatants, and the distribution of nuclear factor kappa B (NF-κB) p65 staining in nucleus, expression of MyD88 and p-IκB in cytoplasm, and DNA-binding activity of NF-κB increased after incubation with PCV2, while p65 expression in PAM cytoplasm decreased. However, when PAMs were co-incubated with PCV2 and small interfering RNA targeting MyD88, those effects were reversed. Additionally, mRNA expression levels of Toll-like receptors (TLR)-2, -3, -4, -7, -8, and -9 increased when PAMs were incubated with PCV2. These results show that PCV2 induces increased IL-1β and IL-10 production in PAMs, and these changes in expression are related to the TLR-MyD88-NF-κB signaling pathway.
Keyword
MeSH Terms
-
Animals
Circoviridae Infections/metabolism/*veterinary/virology
*Circovirus/metabolism
In Vitro Techniques
Interleukin-10/*metabolism
Interleukin-1beta/*metabolism
Macrophages, Alveolar/*metabolism/virology
Myeloid Differentiation Factor 88/*physiology
NF-kappa B/*physiology
*Signal Transduction/physiology
Swine
Swine Diseases/metabolism/virology
Interleukin-1beta
Myeloid Differentiation Factor 88
NF-kappa B
Interleukin-10
Figure
Reference
-
1. Allan GM, Ellis JA. Porcine circoviruses: a review. J Vet Diagn Invest. 2000; 12:3–14.
Article2. Chae JS, Choi KS. Proinflammatory cytokine expression in the lung of pigs with porcine circovirus type 2-associated respiratory disease. Res Vet Sci. 2011; 90:321–323.
Article3. Chang HW, Jeng CR, Lin TL, Liu JJ, Chiou MT, Tsai YC, Chia MY, Jan TR, Pang VF. Immunopathological effects of porcine circovirus type 2 (PCV2) on swine alvelolar macrophages by in vitro inoculation. Vet Immunol Immunopathol. 2006; 110:207–219.
Article4. Chang HW, Pang VF, Chen LJ, Chia MY, Tsai YC, Jeng CR. Bacterial lipopolysaccharide induces porcine circovirus type 2 replication in swine alveolar macrophages. Vet Microbiol. 2006; 115:311–319.
Article5. Darwich L, Pié S, Rovira A, Segalés J, Domingo M, Oswald IP, Mateu E. Cytokine mRNA expression profiles in lymphoid tissues of pigs naturally affected by postweaning multisytemic wasting syndrome. J Gen Virol. 2003; 84:2117–2125.
Article6. Darwich L, Segalés J, Mateu E. Pathogenesis of postweaning multisystemic wasting syndrome caused by Porcine circovirus 2: an immune riddle. Arch Virol. 2004; 149:857–874.
Article7. Duan D, Zhang S, Li X, Guo H, Chen M, Zhang Y, Han J, Lv Y. Activation of the TLR/MyD88/NF-κB signal pathway contributes to changes in IL-4 and IL-12 production in piglet lymphocytes infected with porcine circovirus type 2 in vitro. PloS One. 2014; 9:e97653.8. Ellis JA, Allan G, Krakowka S. Effect of coinfection with genogroup 1 porcine torque teno virus on porcine circovirus type 2-associated postweaning multisystemic wasting syndrome in gnotobiotic pigs. Am J Vet Res. 2008; 69:1608–1614.
Article9. Gilpin DF, McCullough K, Meehan BM, McNeilly F, McNair I, Stevenson LS, Foster JC, Ellis JA, Krakowka S, Adair BM, Allan GM. In vitro studies on the infection and replication of porcine circovirus type 2 in cells of the porcine immune system. Vet Immunol Immunopathol. 2003; 94:149–161.
Article10. Hertzog PJ, O’Neill LA, Hamilton JA. The interferon in TLR signaling: more than just antiviral. Trends Immunol. 2003; 24:534–539.
Article11. Kekarainen T, Montoya M, Mateu E, Segalés J. Porcine circovirus type 2-induced interleukin-10 modulates recall antigen responses. J Gen Virol. 2008; 89:760–765.
Article12. Krakowka S, Ellis J, McNeilly F, Waldner C, Rings DM, Allan G. Mycoplasma hyopneumoniae bacterins and porcine circovirus type 2 (PCV2) infection: induction of postweaning multisystemic wasting syndrome (PMWS) in the gnotobiotic swine model of PCV2-associated disease. Can Vet J. 2007; 48:716–724.13. Li J, Yu Q, Nie X, Guo X, Song Q, Li H. Effects of porcine circovirus type 2 on expression of mRNA associated with endogenous antigen processing and presentation in pulmonary alveolar macrophages and circulating T lymphocytes in piglets. Vet J. 2012; 193:199–205.
Article14. Liu J, Bai J, Lu Q, Zhang L, Jiang Z, Michal JJ, He Q, Jiang P. Two-dimensional liquid chromatography-tandem mass spectrometry coupled with isobaric tags for relative and absolute quantification (iTRAQ) labeling approach revealed first proteome profiles of pulmonary alveolar macrophages infected with porcine circovirus type 2. J Proteomics. 2013; 79:72–86.
Article15. Lv Y, Dai L, Han H, Zhang S. PCV2 induces apoptosis and modulates calcium homeostasis in piglet lymphocytes in vitro. Res Vet Sci. 2012; 93:1525–1530.
Article16. Lv Y, Zhang X, Sun Y, Zhang S. Activation of NF-κB contributes to production of pig-major acute protein and serum amyloid A in pigs experimentally infected with porcine circovirus type 2. Res Vet Sci. 2013; 95:1235–1240.
Article17. Mandrioli L, Sarli G, Panarese S, Baldoni S, Marcato PS. Apoptosis and proliferative activity in lymph node reaction in postweaning multisystemic wasting syndrome (PMWS). Vet Immunol Immunopathol. 2004; 97:25–37.
Article18. Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008; 226:205–218.
Article19. Pang VF, Lambert RJ, Felsburg PJ, Beasley VR, Buck WB, Haschek WM. Experimental T-2 toxicosis in swine following inhalation exposure: effects on pulmonary and systemic immunity, and morphological changes. Toxicol Pathol. 1987; 15:308–319.
Article20. Rovira A, Balasch M, Segalés J, García L, Plana-Durán J, Rosell C, Ellerbrok H, Mankertz A, Domingo M. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J Virol. 2002; 76:3232–3239.
Article21. Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010; 21:315–324.
Article22. Shi KC, Guo X, Ge XN, Liu Q, Yang HC. Cytokine mRNA expression profiles in peripheral blood mononuclear cells from piglets experimentally co-infected with porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Vet Microbiol. 2010; 140:155–160.
Article23. Wei L, Kwang J, Wang J, Shi L, Yang B, Li Y, Liu J. Porcine circovirus type 2 induces the activation of nuclear factors kappa B by IκBα degradation. Virology. 2008; 378:177–184.
Article24. Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi Q, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003; 301:640–643.
Article25. Yu S, Opriessnig T, Kitikoon P, Nilubol D, Halbur PG, Thacker E. Porcine circovirus type 2 (PCV2) distribution and replication in tissues and immune cells in early infected pigs. Vet Immunol Immunopathol. 2007; 115:261–272.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MyD88-BLT2-dependent cascade contributes to LPS-induced interleukin-6 production in mouse macrophage
- Pathologic studies in lymph nodes of pigs infected with porcine circovirus type 2, porcine reproductive and respiratory syndrome virus
- The Role of the Ubiquitin-Proteasome Pathway in Neurodegenerative Disorders
- Activation of Nuclear Factor Kappa B by Inducers in Gestational Tissues at Term
- Effects of B-16 Melanoma Cells and Mycoplasma pneumoniae on the Induction of IL-1 beta, IL-2, IL-6, IL-10, IL-12, and TNF - alpha from Mouse Astrocytes