J Breast Cancer.
2018 Jun;21(2):103-111. 10.4048/jbc.2018.21.2.103.
Knockdown of Chloride Channel-3 Inhibits Breast Cancer Growth In Vitro and In Vivo
- Affiliations
-
- 1Department of Pharmacology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China. tangyb@mail.sysu.edu.cn
- 2Department of Pharmacy, The Fifth Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
- 3Department of Clinical Pharmacology, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
- KMID: 2413928
- DOI: http://doi.org/10.4048/jbc.2018.21.2.103
Abstract
- PURPOSE
Chloride channel-3 (ClC-3) is a member of the chloride channel family and plays a critical role in a variety of cellular activities. The aim of the present study is to explore the molecular mechanisms underlying the antitumor effect of silencing ClC-3 in breast cancer.
METHODS
Human breast cancer cell lines MDA-MB-231 and MCF-7 were used in the experiments. Messenger RNA and protein expression were examined by quantitative real-time polymerase chain reaction and western blot analysis. Cell proliferation was measured by the bromodeoxyuridine method, and the cell cycle was evaluated using fluorescence-activated cell sorting. Protein interaction in cells was analyzed by co-immunoprecipitation. Tumor tissues were stained with hematoxylin-eosin and tumor burden was measured using the Metamorph software.
RESULTS
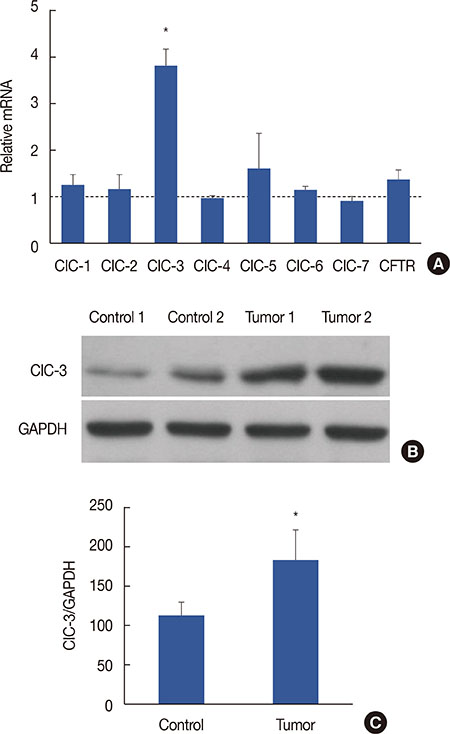
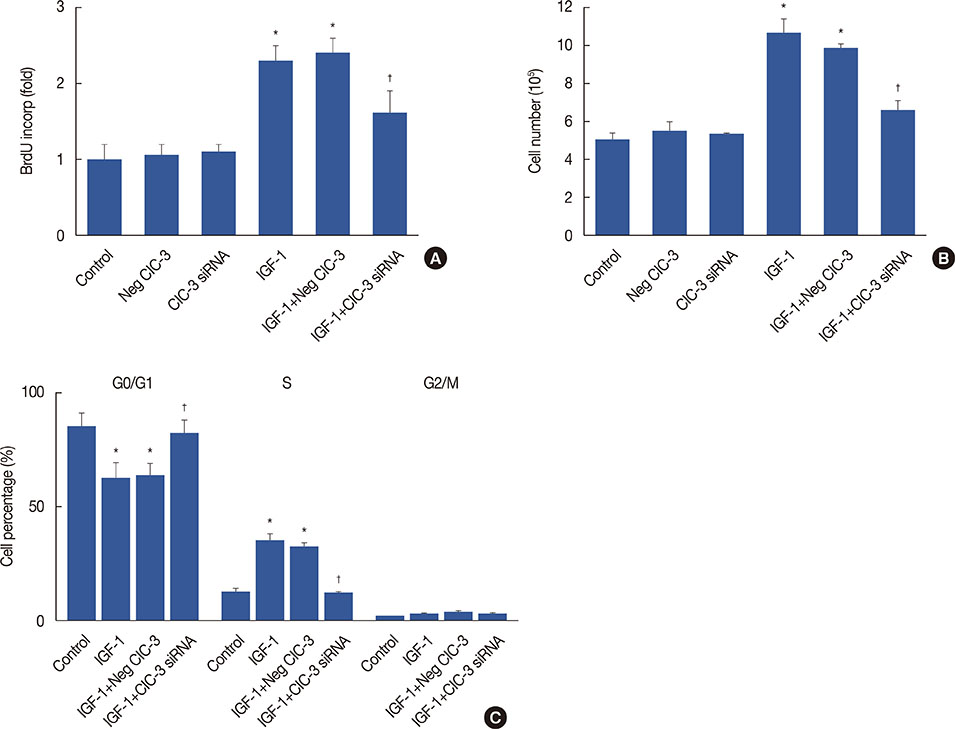
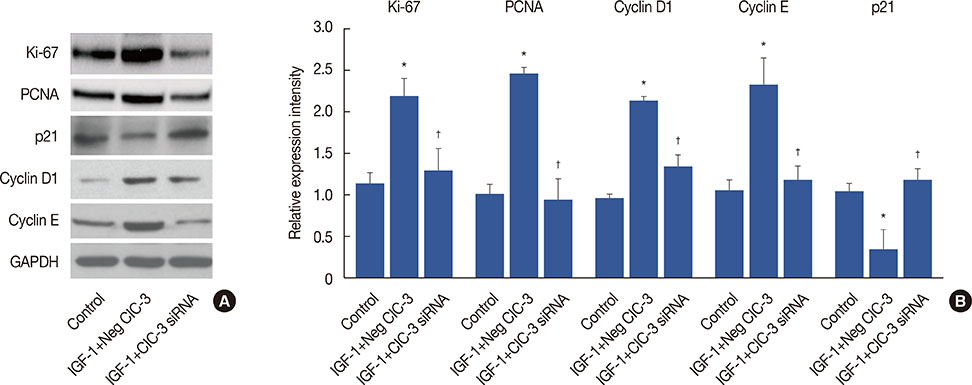
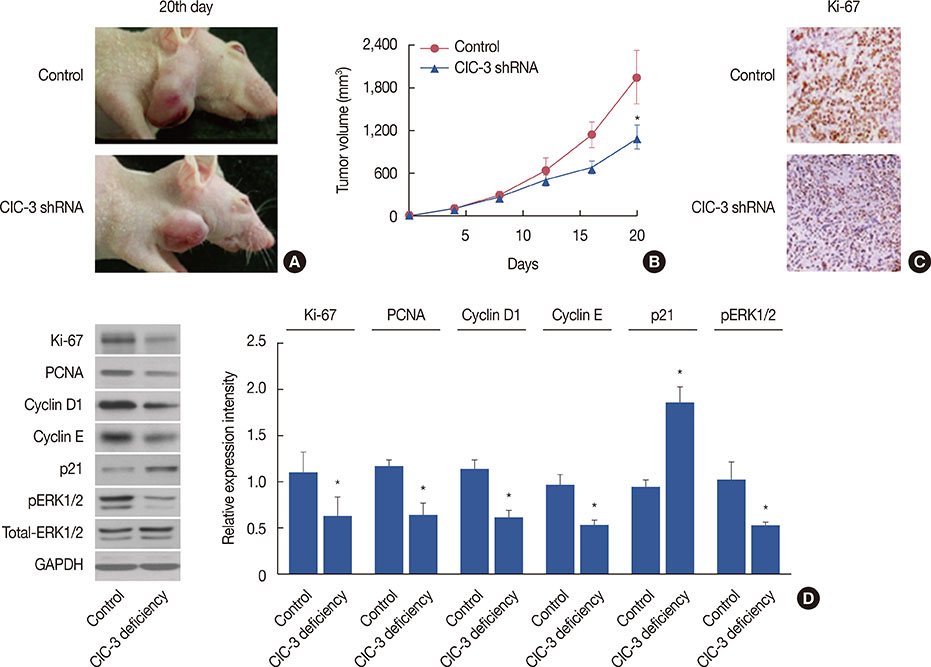
Breast cancer tissues collected from patients showed an increase in ClC-3 expression. Knockdown of ClC-3 inhibited the secretion of insulin-like growth factor (IGF)-1, cell proliferation, and G1/S transition in breast cancer cells. In the mouse xenograft model of human breast carcinoma, tumor growth was significantly slower in animals injected with ClC-3-deficient cells compared with the growth of normal human breast cancer cells. In addition, silencing of ClC-3 attenuated the expression of proliferating cell nuclear antigen, Ki-67, cyclin D1, and cyclin E, as well as the activation of extracellular signal-regulated protein kinases (ERK) 1/2, both in vitro and in vivo.
CONCLUSION
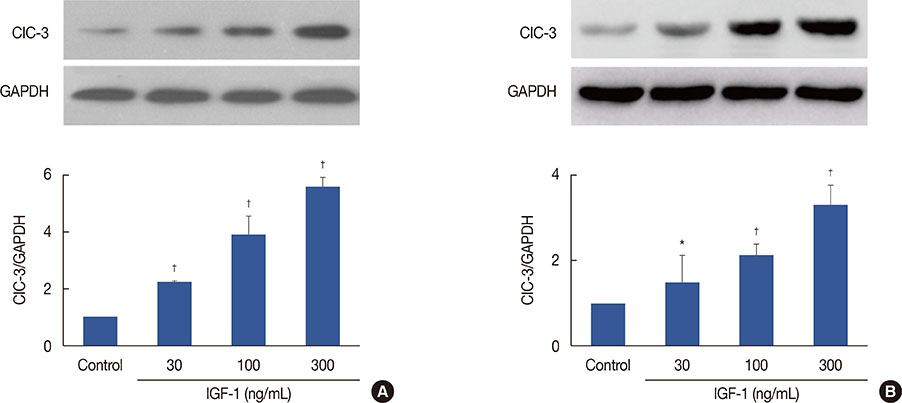
Together, our data suggest that upregulation of ClC-3 by IGF-1 contributes to cell proliferation and tumor growth in breast cancer, and ClC-3 deficiency suppresses cell proliferation and tumor growth via the IGF/IGF receptor/ERK pathway.
MeSH Terms
-
Animals
Blotting, Western
Breast Neoplasms*
Breast*
Bromodeoxyuridine
Cell Cycle
Cell Line
Cell Proliferation
Chloride Channels
Cyclin D1
Cyclin E
Cyclins
Flow Cytometry
Heterografts
Humans
Immunoprecipitation
In Vitro Techniques*
Insulin-Like Growth Factor I
Methods
Mice
Proliferating Cell Nuclear Antigen
Protein Kinases
Real-Time Polymerase Chain Reaction
RNA, Messenger
Tumor Burden
Up-Regulation
Bromodeoxyuridine
Chloride Channels
Cyclin D1
Cyclin E
Cyclins
Insulin-Like Growth Factor I
Proliferating Cell Nuclear Antigen
Protein Kinases
RNA, Messenger
Figure
Reference
-
1. Singh E, Joffe M, Cubasch H, Ruff P, Norris SA, Pisa PT. Breast cancer trends differ by ethnicity: a report from the South African National Cancer Registry (1994-2009). Eur J Public Health. 2017; 27:173–178.
Article2. Kreiter E, Richardson A, Potter J, Yasui Y. Breast cancer: trends in international incidence in men and women. Br J Cancer. 2014; 110:1891–1897.
Article3. Yang H, Ma L, Wang Y, Zuo W, Li B, Yang Y, et al. Activation of ClC-3 chloride channel by 17beta-estradiol relies on the estrogen receptor alpha expression in breast cancer. J Cell Physiol. 2018; 233:1071–1081.
Article4. Xu B, Jin X, Min L, Li Q, Deng L, Wu H, et al. Chloride channel-3 promotes tumor metastasis by regulating membrane ruffling and is associated with poor survival. Oncotarget. 2015; 6:2434–2450.
Article5. Lemonnier L, Lazarenko R, Shuba Y, Thebault S, Roudbaraki M, Lepage G, et al. Alterations in the regulatory volume decrease (RVD) and swelling-activated Cl- current associated with neuroendocrine differentiation of prostate cancer epithelial cells. Endocr Relat Cancer. 2005; 12:335–349.
Article6. Mao J, Chen L, Xu B, Wang L, Li H, Guo J, et al. Suppression of ClC-3 channel expression reduces migration of nasopharyngeal carcinoma cells. Biochem Pharmacol. 2008; 75:1706–1716.
Article7. Du S, Yang L. ClC-3 chloride channel modulates the proliferation and migration of osteosarcoma cells via AKT/GSK3beta signaling pathway. Int J Clin Exp Pathol. 2015; 8:1622–1630.8. Kasinathan RS, Föller M, Lang C, Koka S, Lang F, Huber SM. Oxidation induces ClC-3-dependent anion channels in human leukaemia cells. FEBS Lett. 2007; 581:5407–5412.
Article9. Cuddapah VA, Sontheimer H. Molecular interaction and functional regulation of ClC-3 by Ca2+/calmodulin-dependent protein kinase II (CaMKII) in human malignant glioma. J Biol Chem. 2010; 285:11188–11196.
Article10. Hong S, Bi M, Wang L, Kang Z, Ling L, Zhao C. CLC-3 channels in cancer (review). Oncol Rep. 2015; 33:507–514.
Article11. Wang L, Ma W, Zhu L, Ye D, Li Y, Liu S, et al. ClC-3 is a candidate of the channel proteins mediating acid-activated chloride currents in nasopharyngeal carcinoma cells. Am J Physiol Cell Physiol. 2012; 303:C14–C23.
Article12. Tang YB, Liu YJ, Zhou JG, Wang GL, Qiu QY, Guan YY. Silence of ClC-3 chloride channel inhibits cell proliferation and the cell cycle via G/S phase arrest in rat basilar arterial smooth muscle cells. Cell Prolif. 2008; 41:775–785.
Article13. Mao J, Chen L, Xu B, Wang L, Wang W, Li M, et al. Volume-activated chloride channels contribute to cell-cycle-dependent regulation of HeLa cell migration. Biochem Pharmacol. 2009; 77:159–168.
Article14. Huang YY, Huang XQ, Zhao LY, Sun FY, Chen WL, Du JY, et al. ClC-3 deficiency protects preadipocytes against apoptosis induced by palmitate in vitro and in type 2 diabetes mice. Apoptosis. 2014; 19:1559–1570.
Article15. Huang EW, Xue SJ, Zhang Z, Zhou JG, Guan YY, Tang YB. Vinpocetine inhibits breast cancer cells growth in vitro and in vivo. Apoptosis. 2012; 17:1120–1130.
Article16. Wang M, Tang YB, Ma MM, Chen JH, Hu CP, Zhao SP, et al. TRPC3 channel confers cerebrovascular remodelling during hypertension via transactivation of EGF receptor signalling. Cardiovasc Res. 2016; 109:34–43.
Article17. Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009; 9:153–166.
Article18. Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation. 1998; 98:82–89.19. Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009; 9:400–414.
Article20. Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007; 26:3279–3290.
Article21. Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007; 26:3291–3310.
Article22. Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, et al. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013; 39:935–946.
Article23. Mester J, Redeuilh G. Proliferation of breast cancer cells: regulation, mediators, targets for therapy. Anticancer Agents Med Chem. 2008; 8:872–885.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Zinc Finger Protein 639 Expression Is a Novel Prognostic Determinant in Breast Cancer
- Downstream Neighbor of Son Overexpression is Associated With Breast Cancer Progression and a Poor Prognosis
- Exploring the Role of the KCNK1 Potassium Channel and Its Inhibition Using Quinidine in Treating Head and Neck Squamous Cell Carcinoma
- In Vitro and In Vivo Study on the Effect of Lysosome-associated Protein Transmembrane 4 Beta on the Progression of Breast Cancer
- Diallyl Trisulfide Inhibits Leptin-induced OncogenicSignaling in Human Breast Cancer Cells but Fails toPrevent Chemically-induced Luminal-type Cancer in Rats