J Vet Sci.
2016 Sep;17(3):289-297. 10.4142/jvs.2016.17.3.289.
Immunologic properties of differentiated and undifferentiated mesenchymal stem cells derived from umbilical cord blood
- Affiliations
-
- 1College of Veterinary Medicine, Gyeongsang National University, Jinju 52828, Korea. scyeon@gnu.ac.kr
- 2School of Medicine, Gyeongsang National University, Jinju 52828, Korea.
- 3Division of Applied Life Science, Gyeongsang National University, Jinju 52828, Korea.
- 4Gyeongnam Wildlife Center, Gyeongsang National University, Jinju 52828, Korea.
- 5Adult Stem Cell Research Center, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea.
- 6College of Veterinary Medicine, Western University, Pomona, CA 91766-1854, USA.
- 7Human Biotech Co. Ltd., Jinju 52839, Korea.
- KMID: 2413127
- DOI: http://doi.org/10.4142/jvs.2016.17.3.289
Abstract
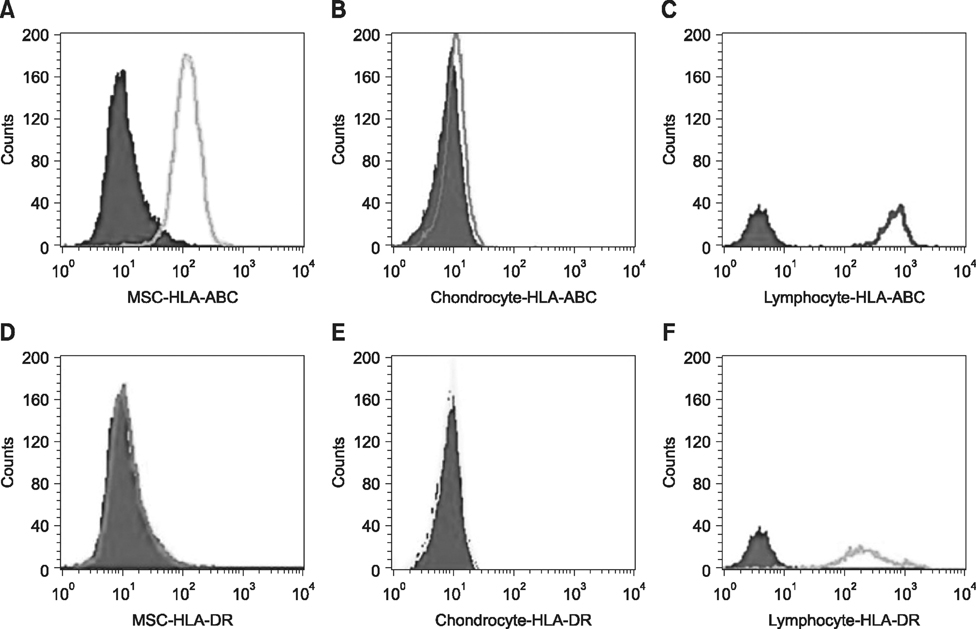
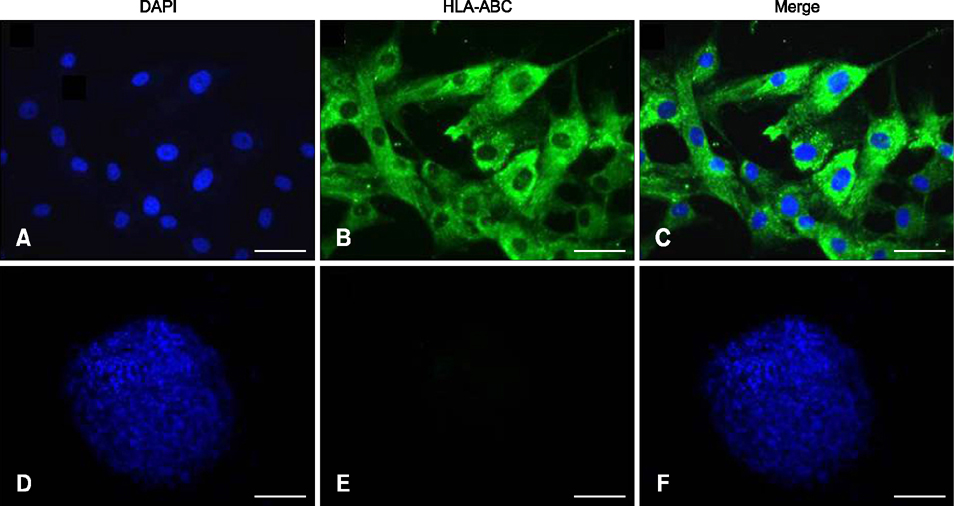
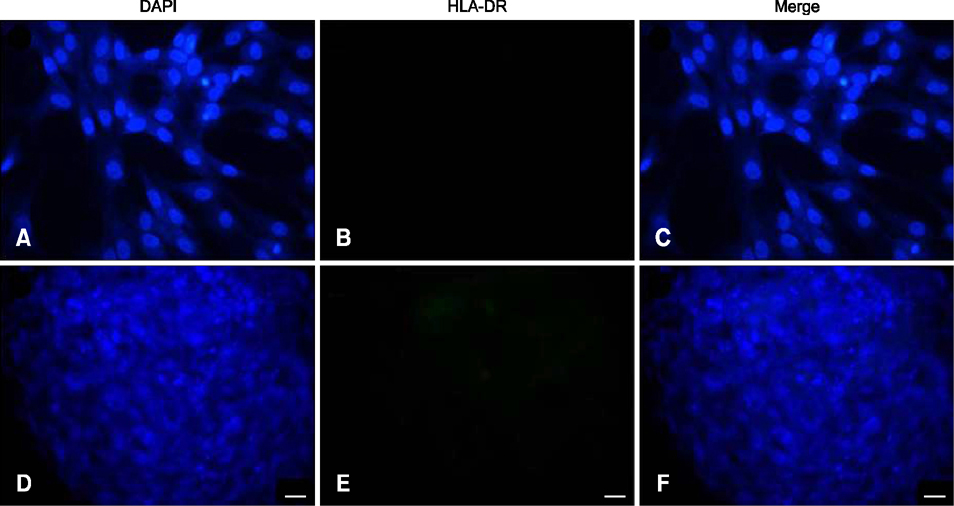
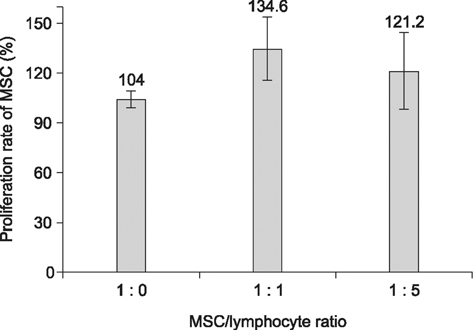
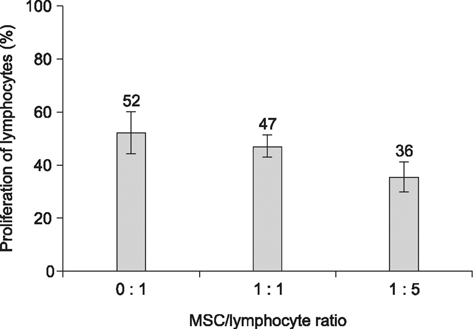
- The expression of immunogenic markers after differentiation of umbilical cord blood (UCB)-derived mesenchymal stem cells (MSC) has been poorly investigated and requires extensive in vitro and in vivo testing for clinical application. The expression of human leukocyte antigen (HLA) classes on UCB-derived MSC was tested by Fluorescence-activated cell sorting analysis and immunocytochemical staining. The undifferentiated MSC were moderately positive for HLA-ABC, but almost completely negative for HLA-DR. The MSC differentiated to chondrocytes expressed neither HLA-ABC nor HLA-DR. The proliferation of MSC was not significantly affected by the allogeneic lymphocytes stimulated with concanavalin A. The responder lymphocytes showed no significant decrease in proliferation in the presence of the MSC, but the apoptosis rate of the lymphocytes was increased in the presence of MSC. Taken together, these findings indicate that UCB-derived MSC differentiated to chondrocytes expressed less HLA class I and no class II antigens. The MSC showed an immunomodulatory effect on the proliferation and apoptosis of allogeneic lymphocytes. These data suggest that the differentiated and undifferentiated allogeneic MSC derived from umbilical cord blood can be a useful candidate for allogeneic cell therapy and transplantation without a major risk of rejection.
Keyword
MeSH Terms
Figure
Reference
-
1. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105:1815–1822.
Article2. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002; 30:42–48.
Article3. Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009; 1176:101–117.
Article4. Chen SL, Fang WW, Qian J, Ye F, Liu YH, Shan SJ, Zhang JJ, Lin S, Liao LM, Zhao RC. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl). 2004; 117:1443–1448.5. Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006; 84:413–421.
Article6. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002; 99:3838–3843.
Article7. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000; 109:235–242.
Article8. Gao K, Chen Y, Wei L, Li S, Jin X, Cong C, Yuan Y, Long D, Li Y, Cheng J, Lu Y. Inhibitory effect of mesenchymal stem cells on lymphocyte proliferation. Cell Biochem Funct. 2008; 26:900–907.
Article9. Götherström C, Ringdén O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004; 190:239–245.
Article10. Groh ME, Maitra B, Szekely E, Koç ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005; 33:928–934.
Article11. Han Q, Sun Z, Liu L, Chen B, Cao Y, Li K, Zhao RC. Impairment in immuno-modulatory function of Flk1+CD31-CD34- MSCs from MDS-RA patients. Leuk Res. 2007; 31:1469–1478.
Article12. Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WWK, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999; 5:309–313.
Article13. Jung DI, Ha J, Kang BT, Kim JW, Quan FS, Lee JH, Woo EJ, Park HM. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J Neurol Sci. 2009; 285:67–77.
Article14. Keating A. How do mesenchymal stromal cells suppress T cells? Cell Stem Cell. 2008; 2:106–108.
Article15. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem cells. 2006; 24:1294–1301.
Article16. Keyser KA, Beagles KE, Kiem HP. Comparison of mesenchymal stem cells from different tissues to suppress T-cell activation. Cell Transplant. 2007; 16:555–562.
Article17. Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005; 12:47–57.
Article18. Koh PO, Cho JH, Nho KH, Cha YI, Kim YK, Cho EH, Lee HC, Jung TS, Yeon SC, Kang KS, Lee HJ. Chondrogenesis of mesenchymal stem cells derived from human umbilical cord blood. J Vet Clin. 2009; 26:528–533.19. Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003; 101:3722–3729.
Article20. Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, Gerson SL, Laughlin MJ, Loberiza FR Jr, Moseley AB, Bacigalupo A. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005; 11:389–398.
Article21. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003; 31:890–896.
Article22. Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003; 57:11–20.
Article23. Lee JH, Chang HS, Kang EH, Chung DJ, Choi CB, Lee JH, Hwang SH, Han H, Kim HY. Percutaneous transplantation of human umbilical cord blood-derived multipotent stem cells in a canine model of spinal cord injury. J Neurosurg Spine. 2009; 11:749–757.
Article24. Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007; 8:275–282.
Article25. Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001; 82:583–590.
Article26. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007; 110:3499–3506.
Article27. Oh W, Kim DS, Yang YS, Lee JK. Immunological properties of umbilical cord blood-derived mesenchymal stromal cells. Cell Immunol. 2008; 251:116–123.
Article28. Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem cells. 2007; 25:2896–2902.
Article29. Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005; 19:1597–1604.
Article30. Prigozhina TB, Khitrin S, Elkin G, Eizik O, Morecki S, Slavin S. Mesenchymal stromal cells lose their immunosuppressive potential after allotransplantation. Exp Hematol. 2008; 36:1370–1376.
Article31. Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005; 129:118–129.
Article32. Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005; 305:33–41.
Article33. Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006; 81:1390–1397.
Article34. Rubinstein P, Rosenfield RE, Adamson JW, Stevens CE. Stored placental blood for unrelated bone marrow reconstitution. Blood. 1993; 81:1679–1690.
Article35. Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS, Lee YW, Kim WH, Kang KS, Kweon OK. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009; 10:273–284.
Article36. Sioud M. New insights into mesenchymal stromal cell-mediated T-cell suppression through galectins. Scand J Immunol. 2011; 73:79–84.
Article37. Technau A, Froelich K, Hagen R, Kleinsasser N. Adipose tissue-derived stem cells show both immunogenic and immunosuppressive properties after chondrogenic differentiation. Cytotherapy. 2011; 13:310–317.
Article38. Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003; 75:389–397.
Article39. Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006; 36:2566–2573.
Article40. Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, Xu J. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009; 126:220–232.
Article41. Yang SE, Ha CW, Jung M, Jin HJ, Lee M, Song H, Choi S, Oh W, Yang YS. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004; 6:476–486.
Article42. Yen BL, Chang CJ, Liu KJ, Chen YC, Hu HI, Bai CH, Yen ML. Brief report—human embryonic stem cell-derived mesenchymal progenitors possess strong immunosuppressive effects toward natural killer cells as well as T lymphocytes. Stem cells. 2009; 27:451–456.
Article43. Zhao Y, Huang Z, Qi M, Lazzarini P, Mazzone T. Immune regulation of T lymphocyte by a newly characterized human umbilical cord blood stem cell. Immunol Lett. 2007; 108:78–87.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Difference in HLA-DR Expression of Human Umbilical Cord Blood Derived Mesenchymal Stem Cells after Tri-lineage Differentiation
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells: Using Explant Method and Umbilical Cord Blood Serum
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Umbilical Cord Derived Mesenchymal Stem Cells Useful in Insulin Production - Another Opportunity in Cell Therapy