J Vet Sci.
2017 Jun;18(2):119-127. 10.4142/jvs.2017.18.2.119.
Neuronal maturation in the hippocampal dentate gyrus via chronic oral administration of Artemisa annua extract is independent of cyclooxygenase 2 signaling pathway in diet-induced obesity mouse model
- Affiliations
-
- 1Department of Biomedical Laboratory Science, College of Medical Sciences, Soonchunhyang University, Asan 31538, Korea. admiral96@sch.ac.kr
- 2Biocenter, Gyeonggi Institute of Science and Technology Promotion (GSTEP), Suwon 16229, Korea.
- 3Department of Medical Biotechnology, College of Medical Sciences, Soonchunhyang University, Asan 31538, Korea.
- 4Department of Biotechnology, Hoseo University, Asan 31499, Korea.
- 5Department of Animal Biotechnology, College of Agricultural Life Science, ChonBuk National University, Jeonju 54896, Korea.
- 6Department of Anatomy and Cell Biology, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea.
- 7Department of Health Science, Konyang University, Nonsan 32992, Korea.
- KMID: 2412564
- DOI: http://doi.org/10.4142/jvs.2017.18.2.119
Abstract
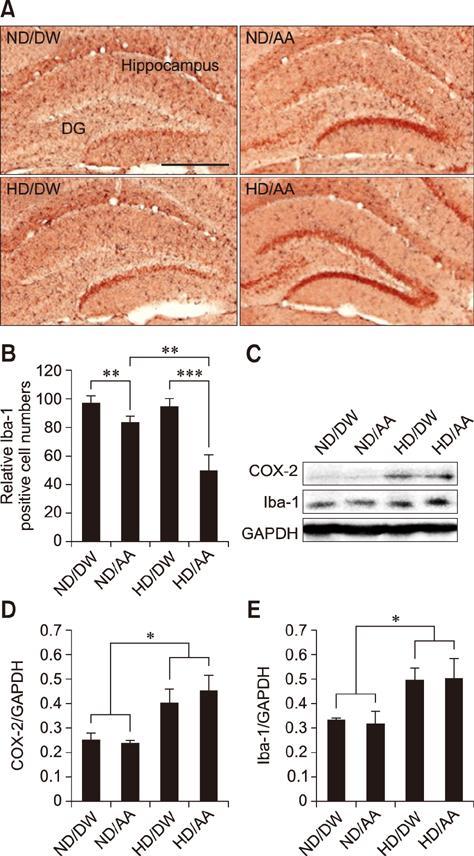
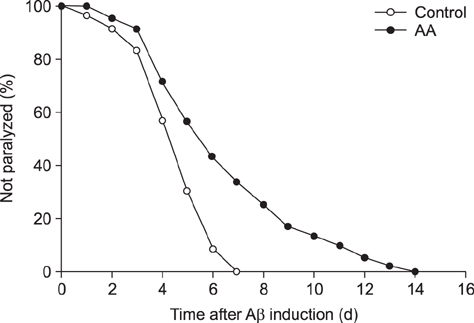
- Recently, we reported that Artemisia annua (AA) has anti-adipogenic properties in vitro and in vivo. Reduction of adipogenesis by AA treatment may dampen systemic inflammation and protect neurons from cytokine-induced damage. Therefore, the present study was undertaken to assess whether AA increases neuronal maturation by reducing inflammatory responses, such as those mediated by cyclooxygenase 2 (COX-2). Mice were fed normal chow or a high-fat diet with or without chronic daily oral administration of AA extract (0.2 g/10 mL/kg) for 4 weeks; then, changes in their hippocampal dentate gyri were measured via immunohistochemistry/immunofluorescence staining for bromodexoxyuridine, doublecortin, and neuronal nuclei, markers of neuronal maturation, and quantitative western blotting for COX-2 and Iba-1, in order to assess correlations between systemic inflammation (interleukin-6) and food type. Additionally, we tested the effect of AA in an Alzheimer's disease model of Caenorhabditis elegans and uncovered a potential benefit. The results show that chronic AA dosing significantly increases neuronal maturation, particularly in the high-fat diet group. This effect was seen in the absence of any changes in COX-2 levels in mice given the same type of food, pointing to the possibility of alternate anti-inflammatory pathways in the stimulation of neurogenesis and neuro-maturation in a background of obesity.
MeSH Terms
-
Adipogenesis/drug effects
Administration, Oral
Animals
Artemisia annua
Cell Differentiation/drug effects
Cyclooxygenase 2/*drug effects
Dentate Gyrus/*drug effects
Diet, High-Fat/adverse effects/veterinary
Fluorescent Antibody Technique/veterinary
Mice
Mice, Inbred C57BL
Neurons/*drug effects
Obesity/metabolism/*veterinary
Plant Extracts/administration & dosage/*pharmacology
Signal Transduction/*drug effects
Plant Extracts
Cyclooxygenase 2
Figure
Reference
-
1. Aral LA, Pinar L, Göktaş G, Deveden EY, Erdoğan D. Comparison of hippocampal interleukin-6 immunoreactivity after exhaustive exercise in both exercise-trained and untrained rats. Turk J Med Sci. 2014; 44:560–568.
Article2. Baek HK, Shim H, Lim H, Shim M, Kim CK, Park SK, Lee YS, Song KD, Kim SJ, Yi SS. Anti-adipogenic effect of Artemisia annua in diet-induced-obesity mice model. J Vet Sci. 2015; 16:389–396.
Article3. Businaro R, Ippoliti F, Ricci S, Canitano N, Fuso A. Alzheimer's disease promotion by obesity: induced mechanisms-molecular links and perspectives. Curr Gerontol Geriatr Res. 2012; 2012:986823.
Article4. Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002; 87:2851–2857.
Article5. Doherty GH. Obesity and the ageing brain: could leptin play a role in neurodegeneration? Curr Gerontol Geriatr Res. 2011; 2011:708154.
Article6. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005; 115:911–919.
Article7. Frühbeck G. Overview of adipose tissue and its role in obesity and metabolic disorders. Methods Mol Biol. 2008; 456:1–22.
Article8. Hoozemans JJ, Rozemuller AJ, Janssen I, De Groot CJ, Veerhuis R, Eikelenboom P. Cyclooxygenase expression in microglia and neurons in Alzheimer's disease and control brain. Acta Neuropathol. 2001; 101:2–8.
Article9. Hwang IK, Yi SS, Yoo KY, Park OK, Yan B, Song W, Won MH, Yoon YS, Seong JK. Differential effects of treadmill exercise in early and chronic diabetic stages on parvalbumin immunoreactivity in the hippocampus of a rat model of type 2 diabetes. Neurochem Res. 2011; 36:1526–1532.
Article10. Lee J, Kim MH, Lee JH, Jung E, Yoo ES, Park D. Artemisinic acid is a regulator of adipocyte differentiation and C/EBP δ expression. J Cell Biochem. 2012; 113:2488–2499.
Article11. Lee MR. Plants against malaria, part 2: Artemisia annua (qinghaosu or the sweet wormwood). J R Coll Physicians Edinb. 2002; 32:300–305.12. Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006; 5:332–353.
Article13. Nam SM, Kim JW, Yoo DY, Choi JH, Kim W, Jung HY, Won MH, Hwang IK, Seong JK, Yoon YS. Comparison of pharmacological and genetic inhibition of cyclooxygenase-2: effects on adult neurogenesis in the hippocampal dentate gyrus. J Vet Sci. 2015; 16:245–251.
Article14. Nam SM, Kim JW, Yoo DY, Choi JH, Kim W, Jung HY, Won MH, Hwang IK, Seong JK, Yoon YS. Effects of treadmill exercise on neural stem cells, cell proliferation, and neuroblast differentiation in the subgranular zone of the dentate gyrus in cyclooxygenase-2 knockout mice. Neurochem Res. 2013; 38:2559–2569.
Article15. Nam SM, Yi SS, Yoo DY, Kim W, Choi JH, Hwang IK, Seong JK, Yoon YS. Changes in cyclooxygenase-2 immunoreactivity in the hippocampus in a model of streptozotocin-induced type 1 diabetic rats. J Vet Med Sci. 2012; 74:977–982.
Article16. Park SK, Park SK. Electrolyzed-reduced water increases resistance to oxidative stress, fertility, and lifespan via insluin/IGF-1-like signal in C. elegans. Biol Res. 2013; 46:147–152.
Article17. Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 3rd ed. Elsevier Science;2004. Figs. 35-52.18. Qian DX, Zhang HT, Ma X, Jiang XD, Xu RX. Comparison of the efficiencies of three neural induction protocols in human adipose stromal cells. Neurochem Res. 2010; 35:572–579.
Article19. Taupin V, Gogusev J, Descamps-Latscha B, Zavala F. Modulation of tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6, interleukin-8, and granulocyte/macrophage colony-stimulating factor expression in human monocytes by an endogenous anxiogenic benzodiazepine ligand, triakontatetraneuropeptide: evidence for a role of prostaglandins. Mol Pharmacol. 1993; 43:64–69.20. Vereyken EJF, Bajova H, Chow S, de Graan PNE, Gruol DL. Chronic interleukin-6 alters the level of synaptic proteins in hippocampus in culture and in vivo. Eur J Neurosci. 2007; 25:3605–3616.
Article21. Willcox M, Falquet J, Ferreira JF, Gilbert B, Hsu E, de Magalhães PM, Plaizier-Vercammen J, Sharma VP, Wright CW, Yaode W. RITAM Artemisia annua Task Force. Artemisia annua as a herbal tea for malaria. Afr J Tradit Complement Altern Med. 2006; 4:121–123.22. Wu B, Hu K, Li S, Zhu J, Gu L, Shen H, Hambly BD, Bao S, Di W. Dihydroartiminisin inhibits the growth and metastasis of epithelial ovarian cancer. Oncol Rep. 2012; 27:101–108.
Article23. Yang H, Zhang J, Andreasson K, Chen C. COX-2 oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity. Mol Cell Neurosci. 2008; 37:682–695.
Article24. Yi SS. Effects of exercise on brain functions in diabetic animal models. World J Diabetes. 2015; 6:583–597.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective Effect of Shenqi-wan on Traumatic Brain Injury-induced Delayed Apoptosis in Rat Hippocampal Dentate Gyrus
- Trimethyltin-Induced Hippocampal Neurodegeneration is Possibly Mediated by Induction of Apoptosis
- The Morphologic Changes of Parvalbumin- Immunoreactive Interneurons of the Dentate Gyrus in Kainate-Treated Mouse Hippocampal Slice Culture Epilepsy Model
- Repeated restraint stress promotes hippocampal neuronal cell ciliogenesis and proliferation in mice
- Vanillin and 4-hydroxybenzyl alcohol attenuate cognitive impairment and the reduction of cell proliferation and neuroblast differentiation in the dentate gyrus in a mouse model of scopolamine-induced amnesia