J Vet Sci.
2017 Sep;18(3):307-316. 10.4142/jvs.2017.18.3.307.
Development of an immunochromatographic strip for detection of antibodies against porcine reproductive and respiratory syndrome virus
- Affiliations
-
- 1College of Veterinary Medicine, Northwest A&F University, Yangling 712100, China. zhanggaiping2003@163.com
- 2Henan Provincial Key Laboratory of Animal Immunology, Henan Academy of Agricultural Sciences, Zhengzhou 450002, China. cdj565@gmail.com
- 3College of Animal Science and Veterinary Medicine, Henan Agricutural University, Zhenzhou 450002, China.
- 4Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonosis, Yangzhou 225009, China.
- 5College of Biology Engineering, Henan University of Animal Husbandry and Economy, Zhengzhou 450046, China.
- KMID: 2412448
- DOI: http://doi.org/10.4142/jvs.2017.18.3.307
Abstract
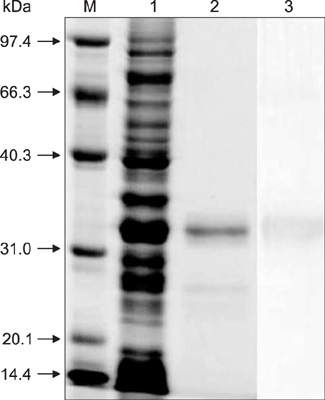
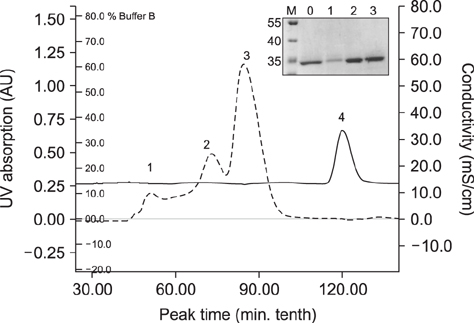
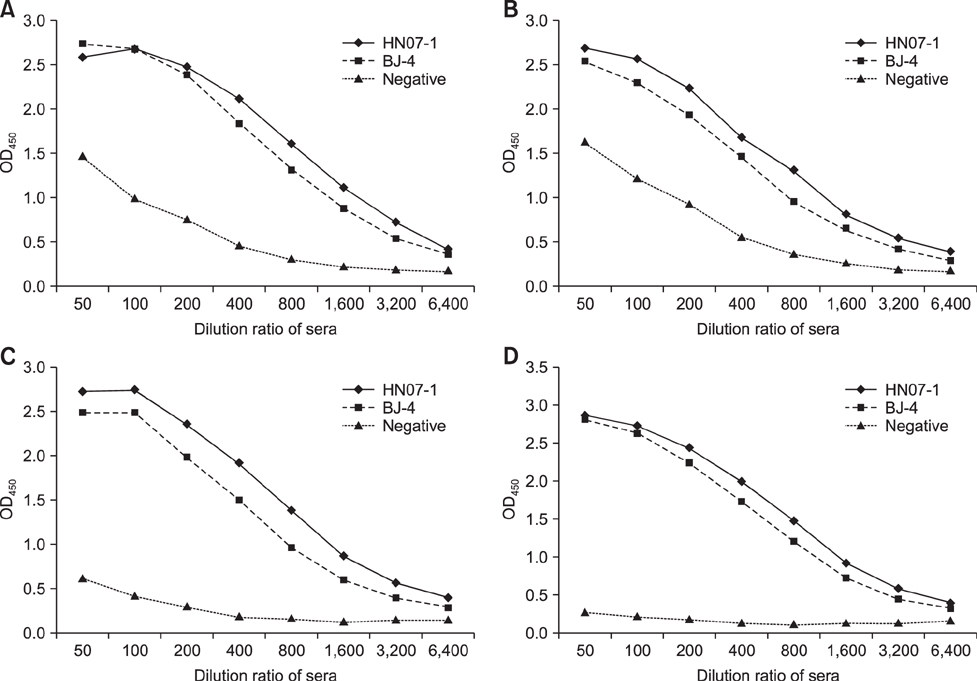
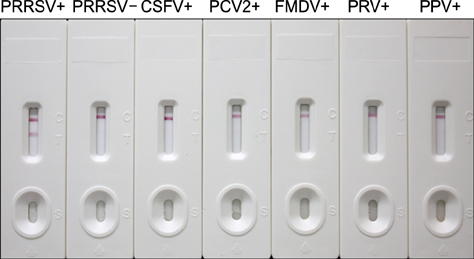
- A simple and rapid immunochromatographic test strip incorporating a colloidal gold-labeled recombinant Nsp7 antigen probe was successfully developed for the detection of anti-porcine reproductive and respiratory syndrome virus (PRRSV) antibodies in swine. Recombinant Nsp7 protein of PRRSV labeled with colloidal gold was dispensed on a conjugate pad for use as the detector. Staphylococcal protein A and purified porcine anti-Nsp7 antibodies were blotted on a nitrocellulose membrane to form test and control lines, respectively. A comparison of the strip with standard diagnostic tests, enzyme-linked immunosorbent assays and immunoperoxidase monolayer assay, was also performed. The immunochromatographic test strip was shown to be of high specificity and sensitivity. Furthermore, the strip assay is rapid and easy to perform with no requirement for professional-level skills or equipment. It is suggested that the immunochromatographic test strip can be used to quickly and accurately detect PRRSV antibody and to be suitable for diagnostic purposes in the field.
Keyword
MeSH Terms
-
Animals
Antibodies, Viral/*immunology
Blotting, Western/veterinary
Enzyme-Linked Immunosorbent Assay/veterinary
Immunochromatography/veterinary
Porcine Reproductive and Respiratory Syndrome/diagnosis/immunology
Porcine respiratory and reproductive syndrome virus/*immunology
*Reagent Strips/therapeutic use
Swine
Antibodies, Viral
Reagent Strips
Figure
Reference
-
1. Albina E, Mesplède A, Chenut G, Le Potier MF, Bourbao G, Le Gal S, Leforban Y. A serological survey on classical swine fever (CSF), Aujeszky's disease (AD) and porcine reproductive and respiratory syndrome (PRRS) virus infections in French wild boars from 1991 to 1998. Vet Microbiol. 2000; 77:43–57.
Article2. Brown E, Lawson S, Welbon C, Gnanandarajah J, Li J, Murtaugh MP, Nelson EA, Molina RM, Zimmerman JJ, Rowland RRR, Fang Y. Antibody response to porcine reproductive and respiratory syndrome virus (PRRSV) nonstructural proteins and implications for diagnostic detection and differentiation of PRRSV types I and II. Clin Vaccine Immunol. 2009; 16:628–635.
Article3. Chen J, Liu T, Zhu CG, Jin YF, Zhang YZ. Genetic variation of Chinese PRRSV strains based on ORF5 sequence. Biochem Genet. 2006; 44:425–435.
Article4. Cho HJ, Deregt D, Joo HS. An ELISA for porcine reproductive and respiratory syndrome: production of antigen of high quality. Can J Vet Res. 1996; 60:89–93.5. Cho HJ, McNab B, Dubuc C, Jordan L, Afshar A, Magar R, Prins S, Eernisse K. Comparative study of serological methods for the detection of antibodies to porcine reproductive and respiratory syndrome virus. Can J Vet Res. 1997; 61:161–166.6. Collins JE, Benfield DA, Christianson WT, Harris L, Hennings JC, Shaw DP, Goyal SM, McCullough S, Morrison RB, Joo HS, Gorcyca D, Chladek Dan. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J Vet Diagn Invest. 1992; 4:117–126.
Article7. Cui S, Zhou S, Chen C, Qi T, Zhang C, Oh J. A simple and rapid immunochromatographic strip test for detecting antibody to porcine reproductive and respiratory syndrome virus. J Virol Methods. 2008; 152:38–42.
Article8. Fang Y, Kim DY, Ropp S, Steen P, Christopher-Hennings J, Nelson EA, Rowland RR. Heterogeneity in Nsp2 of European-like porcine reproductive and respiratory syndrome viruses isolated in the United States. Virus Res. 2004; 100:229–235.
Article9. Fang Y, Schneider P, Zhang WP, Faaberg KS, Nelson EA, Rowland RRR. Diversity and evolution of a newly emerged North American Type 1 porcine arterivirus: analysis of isolates collected between 1999 and 2004. Arch Virol. 2007; 152:1009–1017.
Article10. Fang Y, Snijder EJ. The PRRSV replicase: exploring the multifunctionality of an intriguing set of nonstructural proteins. Virus Res. 2010; 154:61–76.
Article11. Mardassi H, Mounir S, Dea S. Identification of major differences in the nucleocapsid protein genes of a Québec strain and European strains of porcine reproductive and respiratory syndrome virus. J Gen Virol. 1994; 75:681–685.
Article12. Mardassi H, Wilson L, Mounir S, Dea S. Detection of porcine reproductive and respiratory syndrome virus and efficient differentiation between Canadian and European strains by reverse transcription and PCR amplification. J Clin Microbiol. 1994; 32:2197–2203.
Article13. Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008; 177:345–351.
Article14. Meulenberg JJ, Hulst MM, de Meijer EJ, Moonen PL, den Besten A, de Kluyver EP, Wensvoort G, Moormann RJ. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993; 192:62–72.
Article15. Nelsen CJ, Murtaugh MP, Faaberg KS. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J Virol. 1999; 73:270–280.
Article16. Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc. 2005; 227:385–392.
Article17. Qiao S, Feng L, Bao D, Guo J, Wan B, Xiao Z, Yang S, Zhang G. Porcine reproductive and respiratory syndrome virus and bacterial endotoxin act in synergy to amplify the inflammatory response of infected macrophages. Vet Microbiol. 2011; 149:213–220.
Article18. Ropp SL, Wees CEM, Fang Y, Nelson EA, Rossow KD, Bien M, Arndt B, Preszler S, Steen P, Christopher-Hennings J, Collins JE, Benfield DA, Faaberg KS. Characterization of emerging European-like porcine reproductive and respiratory syndrome virus isolates in the United States. J Virol. 2004; 78:3684–3703.
Article19. Spagnuolo-Weaver M, Walker IW, McNeilly F, Calvert V, Graham D, Burns K, Adair BM, Allan GM. The reverse transcription polymerase chain reaction for the diagnosis of porcine reproductive and respiratory syndrome: comparison with virus isolation and serology. Vet Microbiol. 1998; 62:207–215.
Article20. Takashima H, Takai K, Goto S. A modified immunoperoxidase assay for detection of antibody porcine reproductive and respiratory syndrome (PRRS) virus in swine sera. J Vet Med Sci. 1999; 61:195–196.
Article21. Wensvoort G, de Kluyver EP, Pol JM, Wagenaar F, Moormann RJ, Hulst MM, Bloemraad R, den Besten A, Zetstra T, Terpstra C. Lelystad virus, the cause of porcine epidemic abortion and respiratory syndrome: a review of mystery swine disease research at Lelystad. Vet Microbiol. 1992; 33:185–193.
Article22. Xiao S, Chen Y, Wang L, Gao J, Mo D, He Z, Liu X. Simultaneous detection and differentiation of highly virulent and classical Chinese-type isolation of PRRSV by real-time RT-PCR. J Immunol Res. 2014; 2014:809656.
Article23. Yang S, Yang J, Zhang G, Wang X, Qiao S, Zhao D, Zhi Y, Li X, Xing G, Luo J, Fan J, Bao D. Development of an immunochromatographic strip for the detection of antibodies against foot-and-mouth disease virus serotype O. J Virol Methods. 2010; 165:139–144.
Article24. Zhang G, Wang X, Zhi A, Bao Y, Yang Y, Qu M, Luo J, Li Q, Guo J, Wang Z, Yang J, Xing G, Chai S, Shi T, Liu Q. Development of a lateral flow immunoassay strip for screening of sulfamonomethoxine residues. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008; 25:413–423.
Article25. Zhang GP, Guo JQ, Wang XN, Yang JX, Yang YY, Li QM, Li XW, Deng RG, Xiao ZJ, Yang JF, Xing GX, Zhao D. Development and evaluation of an immunochromatographic strip for trichinellosis detection. Vet Parasitol. 2006; 137:286–293.
Article26. Zhang GP, Wang XN, Yang JF, Yang YY, Xing GX, Li QM, Zhao D, Chai SJ, Guo JQ. Development of an immunochromatographic lateral flow test strip for detection of beta-adrenergic agonist Clenbuterol residues. J Immunol Methods. 2006; 312:27–33.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A rapid and quantitative fluorescent microsphere immunochromatographic strip test for detection of antibodies to porcine reproductive and respiratory syndrome virus
- An enhanced immunochromatographic strip test using colloidal gold nanoparticle-labeled dual-type N proteins for detection of antibodies to PRRS virus
- Age-specific Prevalence of Porcine Reproductive and Respiratory Syndrome Virus, Porcine Circovirus Type 2, and Mycoplasma hyopneumoniae in Korea Pig Farms
- Prevalence of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2 and porcine parvovirus from aborted fetuses and pigs with respiratory problems in Korea
- Augmented immune responses in pigs immunized with an inactivated porcine reproductive and respiratory syndrome virus containing the deglycosylated glycoprotein 5 under field conditions