Clin Exp Vaccine Res.
2016 Jan;5(1):70-74. 10.7774/cevr.2016.5.1.70.
Augmented immune responses in pigs immunized with an inactivated porcine reproductive and respiratory syndrome virus containing the deglycosylated glycoprotein 5 under field conditions
- Affiliations
-
- 1College of Veterinary Medicine, Konkuk University, Seoul, Korea. odssey@konkuk.ac.kr
- 2Division of Vaccine Research, Korea National Institute of Health, Korea Centers for Disease Control and Prevention, Cheongju, Korea.
- KMID: 2152699
- DOI: http://doi.org/10.7774/cevr.2016.5.1.70
Abstract
- PURPOSE
Porcine reproductive and respiratory syndrome virus (PRRSV) leads to major economic losses in the swine industry. Vaccination is the most effective method to control the disease by PRRSV.
MATERIALS AND METHODS
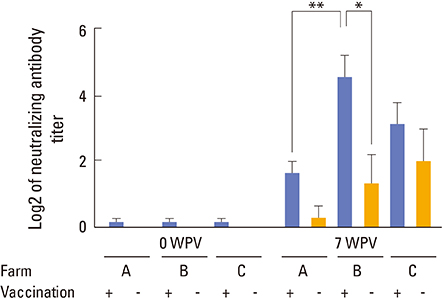
In this study, the efficacy of a glycoprotein (GP) 5-modified inactivated vaccine was investigated in pigs. The study was performed in three farms: farm A, which was porcine reproductive and respiratory syndrome (PRRS)-negative, farm B (PRRS-active), which showed clinical signs of PRRS but had not used vaccines, and farm C (PRRS-stable), which had a history of endemic PRRS over the past years, but showed no more clinical signs after periodic administration of modified live virus vaccine.
RESULTS
The inactivated vaccine induced great enhancement in serum neutralizing antibody titer, which was sufficient to protect pigs from further infections of PRRSV in a farm where pre-existing virus was circulating.
CONCLUSION
These results indicated that vaccination with the inactivated vaccine composed of viruses possessing deglycosylated GP5 would provide enhanced protection to pigs from farms suffering from endemic PRRSV.
Keyword
MeSH Terms
Figure
Reference
-
1. Neumann EJ, Kliebenstein JB, Johnson CD, et al. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc. 2005; 227:385–392.
Article2. Charerntantanakul W. Porcine reproductive and respiratory syndrome virus vaccines: Immunogenicity, efficacy and safety aspects. World J Virol. 2012; 1:23–30.
Article3. Nielsen HS, Oleksiewicz MB, Forsberg R, Stadejek T, Botner A, Storgaard T. Reversion of a live porcine reproductive and respiratory syndrome virus vaccine investigated by parallel mutations. J Gen Virol. 2001; 82(Pt 6):1263–1272.
Article4. Murtaugh MP, Stadejek T, Abrahante JE, Lam TT, Leung FC. The ever-expanding diversity of porcine reproductive and respiratory syndrome virus. Virus Res. 2010; 154:18–30.
Article5. Bassaganya-Riera J, Thacker BJ, Yu S, Strait E, Wannemuehler MJ, Thacker EL. Impact of immunizations with porcine reproductive and respiratory syndrome virus on lymphoproliferative recall responses of CD8+ T cells. Viral Immunol. 2004; 17:25–37.
Article6. Kim H, Kim HK, Jung JH, et al. The assessment of efficacy of porcine reproductive respiratory syndrome virus inactivated vaccine based on the viral quantity and inactivation methods. Virol J. 2011; 8:323.
Article7. Plana Duran J, Climent I, Sarraseca J, et al. Baculovirus expression of proteins of porcine reproductive and respiratory syndrome virus strain Olot/91: involvement of ORF3 and ORF5 proteins in protection. Virus Genes. 1997; 14:19–29.8. Barfoed AM, Blixenkrone-Moller M, Jensen MH, Botner A, Kamstrup S. DNA vaccination of pigs with open reading frame 1-7 of PRRS virus. Vaccine. 2004; 22:3628–3641.
Article9. Cruz JL, Zuniga S, Becares M, et al. Vectored vaccines to protect against PRRSV. Virus Res. 2010; 154:150–160.
Article10. Prieto C, Martinez-Lobo FJ, Diez-Fuertes F, Aguilar-Calvo P, Simarro I, Castro JM. Immunisation of pigs with a major envelope protein sub-unit vaccine against porcine reproductive and respiratory syndrome virus (PRRSV) results in enhanced clinical disease following experimental challenge. Vet J. 2011; 189:323–329.
Article11. Lee JA, Kwon B, Osorio FA, et al. Protective humoral immune response induced by an inactivated porcine reproductive and respiratory syndrome virus expressing the hypo-glycosylated glycoprotein 5. Vaccine. 2014; 32:3617–3622.
Article12. Lee JA, Lee NH, Lee SW, et al. Development of a chimeric strain of porcine reproductive and respiratory syndrome virus with an infectious clone and a Korean dominant field strain. J Microbiol. 2014; 52:345–349.
Article13. Lee JA, Lee NH, Lee JB, et al. Genetic diversity of the Korean field strains of porcine reproductive and respiratory syndrome virus. Infect Genet Evol. 2015; 11. 03. [Epub]. DOI: 10.1016/j.meegid.2015.11.001.
Article14. Yoon IJ, Joo HS, Goyal SM, Molitor TW. A modified serum neutralization test for the detection of antibody to porcine reproductive and respiratory syndrome virus in swine sera. J Vet Diagn Invest. 1994; 6:289–292.
Article15. Ansari IH, Kwon B, Osorio FA, Pattnaik AK. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J Virol. 2006; 80:3994–4004.
Article16. Jiang W, Jiang P, Wang X, Li Y, Wang X, Du Y. Influence of porcine reproductive and respiratory syndrome virus GP5 glycoprotein N-linked glycans on immune responses in mice. Virus Genes. 2007; 35:663–671.
Article17. Mavromatis I, Kritas SK, Alexopoulos C, Tsinas A, Kyriakis SC. Field evaluation of a live vaccine against porcine reproductive and respiratory syndrome in fattening pigs. Zentralbl Veterinarmed B. 1999; 46:603–612.
Article18. Scortti M, Prieto C, Alvarez E, Simarro I, Castro JM. Failure of an inactivated vaccine against porcine reproductive and respiratory syndrome to protect gilts against a heterologous challenge with PRRSV. Vet Rec. 2007; 161:809–813.19. Zuckermann FA, Garcia EA, Luque ID, et al. Assessment of the efficacy of commercial porcine reproductive and respiratory syndrome virus (PRRSV) vaccines based on measurement of serologic response, frequency of gamma-IFN-producing cells and virological parameters of protection upon challenge. Vet Microbiol. 2007; 123:69–85.
Article20. Papatsiros VG, Alexopoulos C, Kritas SK, et al. Long-term administration of a commercial porcine reproductive and respiratory syndrome virus (PRRSV)-inactivated vaccine in PRRSV-endemically infected sows. J Vet Med B Infect Dis Vet Public Health. 2006; 53:266–272.
Article21. Kritas SK, Alexopoulos C, Kyriakis CS, Tzika E, Kyriakis SC. Performance of fattening pigs in a farm infected with both porcine reproductive and respiratory syndrome (PRRS) virus and porcine circovirus type 2 following sow and piglet vaccination with an attenuated PRRS vaccine. J Vet Med A Physiol Pathol Clin Med. 2007; 54:287–291.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Porcine reproductive and respiratory syndrome virus vaccine does not fit in classical vaccinology
- Evaluation of the efficacy of an attenuated live vaccine based on virulent porcine reproductive and respiratory syndrome virus 2 in young pigs
- Pathologic studies in lymph nodes of pigs infected with porcine circovirus type 2, porcine reproductive and respiratory syndrome virus
- Porcine ear necrosis syndrome by coinfection of porcine reproductive and respiratory syndrome virus and Staphylococcus hyicus
- Prevalence of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2 and porcine parvovirus from aborted fetuses and pigs with respiratory problems in Korea