Cancer Res Treat.
2018 Apr;50(2):335-344. 10.4143/crt.2017.070.
Clinical Outcomes of Proton Beam Therapy for Choroidal Melanoma at a Single Institute in Korea
- Affiliations
-
- 1Department of Ophthalmology, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Radiation Oncology, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea.
- 3Department of Radiation Oncology, Baylor Scott & White Health, Temple, TX, USA.
- 4Proton Therapy Center, Research Institute and Hospital, National Cancer Center, Goyang, Korea. shmoon@ncc.re.kr
- 5Ophthalmology Clinic, Research Institute and Hospital, National Cancer Center, Goyang, Korea.
- 6Yonsei Jang's Eye Clinic, Seoul, Korea.
- KMID: 2411123
- DOI: http://doi.org/10.4143/crt.2017.070
Abstract
- PURPOSE
This study retrospectively evaluated the clinical outcomes and complications of proton beam therapy (PBT) in a single institution in Korea and quantitatively analyzed the change in tumor volume after PBT using magnetic resonance imaging (MRI).
MATERIALS AND METHODS
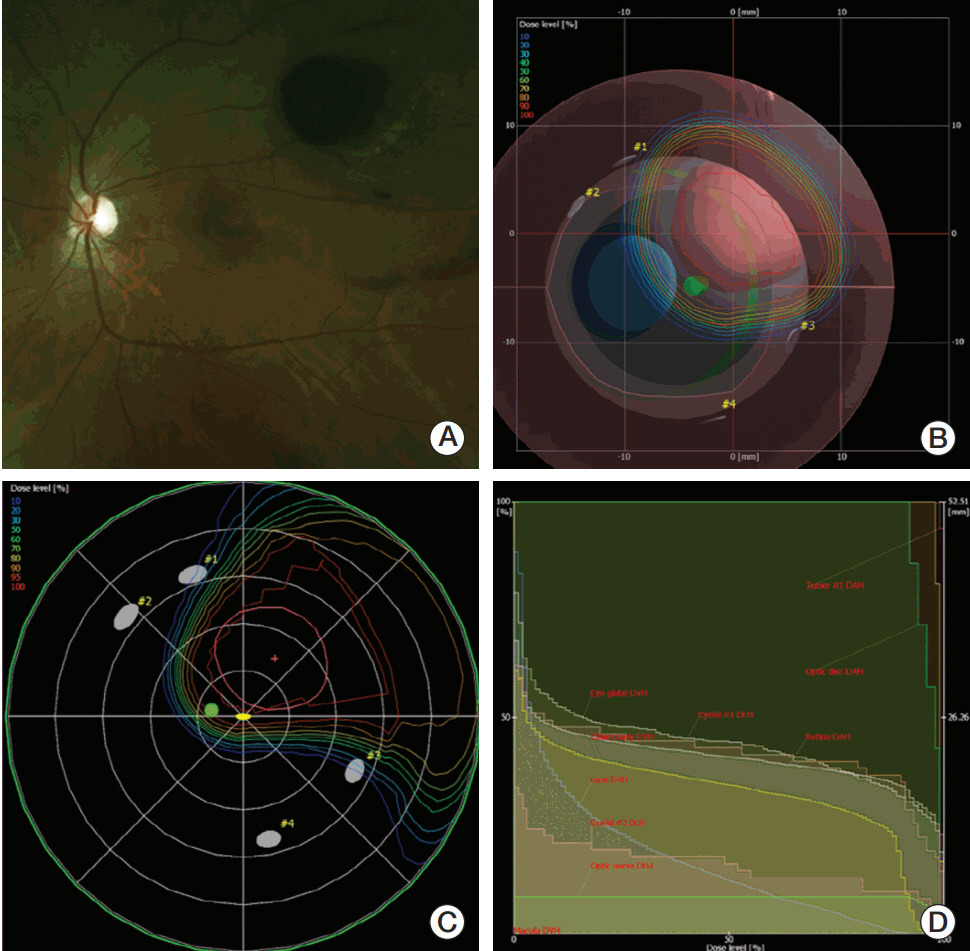
Twenty-four treatment-naïve patients who underwent PBT for choroidal melanoma between 2009 and 2015 were reviewed. Dose fractionation was 60-70 cobalt gray equivalents over 5 fractions. Orbital MRIs were taken at baseline and 3, 6, and 12 months after PBT and annually thereafter. The tumor volume was reconstructed and evaluated by stacking the tumor boundary in each thin-sliced axial T1-weighted image using MIM software.
RESULTS
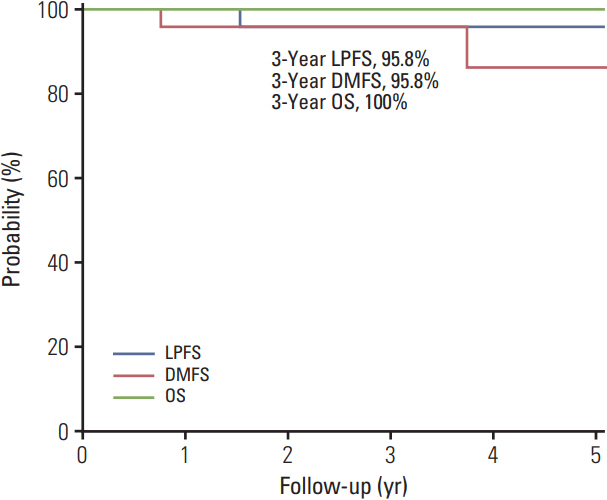
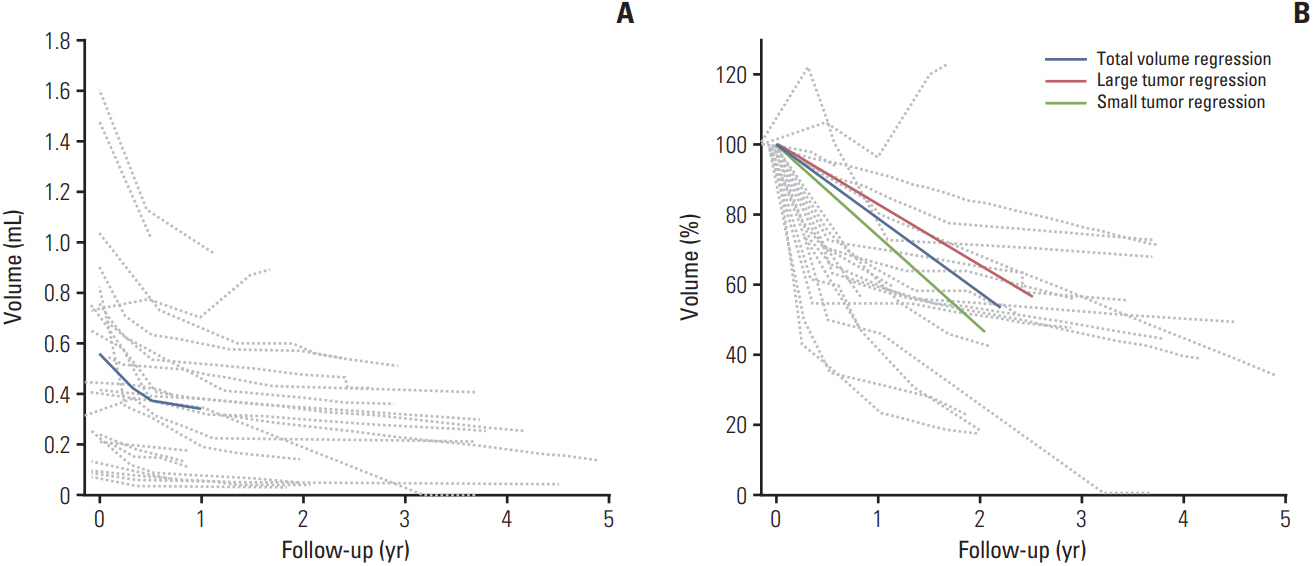
The median follow-up duration was 36.5 months (range, 9 to 82 months). One patient had suspicious local progression and two patients had distant metastasis. The 3-year local progression-free survival, distant metastasis-free survival, and overall survival rates were 95.8%, 95.8%, and 100%,respectively. Five Common Terminology Criteria for Adverse Event ver. 4.03 grade 3-4 toxicities were observed in four patients (16.7%), including one with neovascular glaucoma. The mean tumor volume at the baseline MRI was 0.565±0.084 mL (range, 0.074 to 1.610 mL), and the ratios of the mean volume at 3, 6, and 12 months to that at baseline were 81.8%, 67.3%, and 60.4%, respectively.
CONCLUSION
The local controlrate and complication profile after PBT in patientswith choroidal melanoma in Korea were comparable with those reported in a previous PBT series. The change in tumor volume after PBT exhibited a gradual regression pattern on MRI.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Who Will Benefit from Charged-Particle Therapy?
Kyung Su Kim, Hong-Gyun Wu
Cancer Res Treat. 2021;53(3):621-634. doi: 10.4143/crt.2021.299.
Reference
-
References
1. Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, et al. Incidence of uveal melanoma in Europe. Ophthalmology. 2007; 114:2309–15.
Article2. Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011; 118:1881–5.
Article3. Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Screening for metastasis from choroidal melanoma: the Collaborative Ocular Melanoma Study Group Report 23. J Clin Oncol. 2004; 22:2438–44.
Article4. Shields JA, Shields CL. Management of posterior uveal melanoma: past, present, and future: the 2014 Charles L. Schepens lecture. Ophthalmology. 2015; 122:414–28.5. Gragoudas ES, Lane AM, Munzenrider J, Egan KM, Li W. Long-term risk of local failure after proton therapy for choroidal/ciliary body melanoma. Trans Am Ophthalmol Soc. 2002; 100:43–8.6. Gragoudas E, Li W, Goitein M, Lane AM, Munzenrider JE, Egan KM. Evidence-based estimates of outcome in patients irradiated for intraocular melanoma. Arch Ophthalmol. 2002; 120:1665–71.
Article7. Egger E, Schalenbourg A, Zografos L, Bercher L, Boehringer T, Chamot L, et al. Maximizing local tumor control and survival after proton beam radiotherapy of uveal melanoma. Int J Radiat Oncol Biol Phys. 2001; 51:138–47.
Article8. Kaneko A. Malignant ophthalmic tumors. Nihon Rinsho. 1993; 51 Suppl:1013–20.9. Kuo PK, Puliafito CA, Wang KM, Liu HS, Wu BF. Uveal melanoma in China. Int Ophthalmol Clin. 1982; 22:57–71.
Article10. Sakamoto T, Sakamoto M, Yoshikawa H, Hata Y, Ishibashi T, Ohnishi Y, et al. Histologic findings and prognosis of uveal malignant melanoma in japanese patients. Am J Ophthalmol. 1996; 121:276–83.
Article11. Kwon HJ, Ko JS, Kim M, Lee CS, Lee SC. Prognosis of choroidal melanoma and the result of ruthenium brachytherapy combined with transpupillary thermotherapy in Korean patients. Br J Ophthalmol. 2013; 97:653–8.
Article12. Shah PK, Selvaraj U, Narendran V, Guhan P, Saxena SK, Dash A. Indigenous (125)I brachytherapy source for the management of intraocular melanomas in India. Cancer Biother Radiopharm. 2013; 28:21–8.
Article13. Tsuji H, Ishikawa H, Yanagi T, Hirasawa N, Kamada T, Mizoe JE, et al. Carbon-ion radiotherapy for locally advanced or unfavorably located choroidal melanoma: a Phase I/II dose-escalation study. Int J Radiat Oncol Biol Phys. 2007; 67:857–62.
Article14. Toyama S, Tsuji H, Mizoguchi N, Nomiya T, Kamada T, Tokumaru S, et al. Long-term results of carbon ion radiation therapy for locally advanced or unfavorably located choroidal melanoma: usefulness of CT-based 2-port orthogonal therapy for reducing the incidence of neovascular glaucoma. Int J Radiat Oncol Biol Phys. 2013; 86:270–6.
Article15. Maschi C, Thariat J, Herault J, Caujolle JP. Tumour response in uveal melanomas treated with proton beam therapy. Clin Oncol (R Coll Radiol). 2016; 28:198–203.
Article16. Russo A, Mariotti C, Longo A, Foti PV, Avitabile T, Uva MG, et al. Diffusion-weighted magnetic resonance imaging and ultrasound evaluation of choroidal melanomas after proton-beam therapy. Radiol Med. 2015; 120:634–40.
Article17. Egan KM, Gragoudas ES, Seddon JM, Glynn RJ, Munzenreider JE, Goitein M, et al. The risk of enucleation after proton beam irradiation of uveal melanoma. Ophthalmology. 1989; 96:1377–82.18. Gragoudas ES. Proton beam irradiation of uveal melanomas: the first 30 years. The Weisenfeld Lecture. Invest Ophthalmol Vis Sci. 2006; 47:4666–73.
Article19. Gragoudas ES, Marie Lane A. Uveal melanoma: proton beam irradiation. Ophthalmol Clin North Am. 2005; 18:111–8.
Article20. Shin D, Yoo SH, Moon SH, Yoon M, Lee SB, Park SY. Eye tracking and gating system for proton therapy of orbital tumors. Med Phys. 2012; 39:4265–73.
Article21. Jampol LM, Moy CS, Murray TG, Reynolds SM, Albert DM, Schachat AP, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology. 2002; 109:2197–206.
Article22. Shields JA, Augsburger JJ, Brady LW, Day JL. Cobalt plaque therapy of posterior uveal melanomas. Ophthalmology. 1982; 89:1201–7.
Article23. Lommatzsch PK. Beta-Irradiation of choroidal melanoma with 106Ru/106Rh applicators. 16 Years' experience. Arch Ophthalmol. 1983; 101:713–7.24. Packer S, Rotman M. Radiotherapy of choroidal melanoma with iodine-125. Ophthalmology. 1980; 87:582–90.
Article25. Gragoudas ES, Egan KM, Saornil MA, Walsh SM, Albert DM, Seddon JM. The time course of irradiation changes in proton beam-treated uveal melanomas. Ophthalmology. 1993; 100:1555–9.
Article26. Sikuade MJ, Salvi S, Rundle PA, Errington DG, Kacperek A, Rennie IG. Outcomes of treatment with stereotactic radiosurgery or proton beam therapy for choroidal melanoma. Eye (Lond). 2015; 29:1194–8.
Article27. Mishra KK, Quivey JM, Daftari IK, Weinberg V, Cole TB, Patel K, et al. Long-term results of the UCSF-LBNL randomized trial: charged particle with helium ion versus Iodine-125 plaque therapy for choroidal and ciliary body melanoma. Int J Radiat Oncol Biol Phys. 2015; 92:376–83.
Article28. Browne AW, Dandapani SV, Jennelle R, Stevanovic M, Lee TC, Murphree AL, et al. Outcomes of medium choroidal melanomas treated with ruthenium brachytherapy guided by three-dimensional pretreatment modeling. Brachytherapy. 2015; 14:718–25.
Article29. Wang Z, Nabhan M, Schild SE, Stafford SL, Petersen IA, Foote RL, et al. Charged particle radiation therapy for uveal melanoma: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2013; 86:18–26.
Article30. Hocht S, Bechrakis NE, Nausner M, Kreusel KM, Kluge H, Heese J, et al. Proton therapy of uveal melanomas in Berlin: 5 years of experience at the Hahn-Meitner Institute. Strahlenther Onkol. 2004; 180:419–24.31. Summanen P, Immonen I, Kivela T, Tommila P, Heikkonen J, Tarkkanen A. Radiation related complications after ruthenium plaque radiotherapy of uveal melanoma. Br J Ophthalmol. 1996; 80:732–9.
Article32. Foss AJ, Whelehan I, Hungerford JL, Anderson DF, Errington RD, Kacperek A, et al. Predictive factors for the development of rubeosis following proton beam radiotherapy for uveal melanoma. Br J Ophthalmol. 1997; 81:748–54.
Article33. Suit HD, Goitein M, Tepper J, Koehler AM, Schmidt RA, Schneider R. Explorotory study of proton radiation therapy using large field techniques and fractionated dose schedules. Cancer. 1975; 35:1646–57.34. Gragoudas ES, Seddon JM, Egan KM, Glynn RJ, Goitein M, Munzenrider J, et al. Metastasis from uveal melanoma after proton beam irradiation. Ophthalmology. 1988; 95:992–9.35. Bedikian AY, Legha SS, Mavligit G, Carrasco CH, Khorana S, Plager C, et al. Treatment of uveal melanoma metastatic to the liver: a review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer. 1995; 76:1665–70.
Article36. Berry JL, Dandapani SV, Stevanovic M, Lee TC, Astrahan M, Murphree AL, et al. Outcomes of choroidal melanomas treated with eye physics: a 20-year review. JAMA Ophthalmol. 2013; 131:1435–42.37. Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003; 44:4651–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proton Therapy Review: Proton Therapy from a Medical
- A Pilot Study of the Scanning Beam Quality Assurance Using Machine Log Files in Proton Beam Therapy
- Therapeutic Proton Beam Range Measurement with EBT3 Film and Comparison with Tool for Particle Simulation
- Proton therapy: the current status of the clinical evidences
- Proton Beam Therapy