Korean J Pain.
2018 Apr;31(2):73-79. 10.3344/kjp.2018.31.2.73.
Can oliceridine (TRV130), an ideal novel µ receptor G protein pathway selective (µ-GPS) modulator, provide analgesia without opioid-related adverse reactions?
- Affiliations
-
- 1Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Korea. pain@pusan.ac.kr
- 2Department of Pain Medicine, The University of Texas M.D. Anderson Cancer Center, Houston, TX, USA.
- KMID: 2410828
- DOI: http://doi.org/10.3344/kjp.2018.31.2.73
Abstract
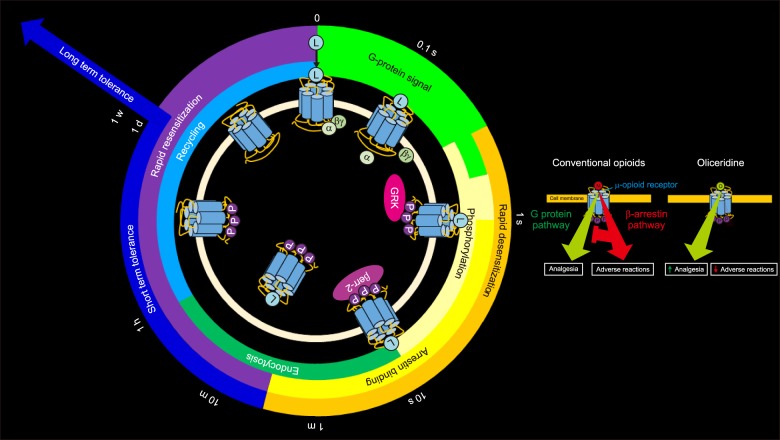
- All drugs have both favorable therapeutic and untoward adverse effects. Conventional opioid analgesics possess both analgesia and adverse reactions, such as nausea, vomiting, and respiratory depression. The opioid ligand binds to µ opioid receptor and non-selectively activates two intracellular signaling pathways: the G protein pathway induce analgesia, while the β-arrestin pathway is responsible for the opioid-related adverse reactions. An ideal opioid should activate the G protein pathway while deactivating the β-arrestin pathway. Oliceridine (TRV130) has a novel characteristic mechanism on the action of the µ receptor G protein pathway selective (µ-GPS) modulation. Even though adverse reactions (ADRs) are significantly attenuated, while the analgesic effect is augmented, the some residual ADRs persist. Consequently, a G protein biased µ opioid ligand, oliceridine, improves the therapeutic index owing to increased analgesia with decreased adverse events. This review article provides a brief history, mechanism of action, pharmacokinetics, pharmacodynamics, and ADRs of oliceridine.
Keyword
MeSH Terms
-
Analgesia
Analgesics, Opioid
Animals
Bias (Epidemiology)
Drug-Related Side Effects and Adverse Reactions
GTP-Binding Proteins*
Intracellular Signaling Peptides and Proteins
Ligands
Mice
Mice, Knockout
Nausea
Patient Safety
Pharmacokinetics
Receptors, Opioid
Receptors, Opioid, mu
Respiratory Insufficiency
Vomiting
Analgesics, Opioid
GTP-Binding Proteins
Intracellular Signaling Peptides and Proteins
Ligands
Receptors, Opioid
Receptors, Opioid, mu
Figure
Cited by 1 articles
-
All about pain pharmacology: what pain physicians should know
Kyung-Hoon Kim, Hyo-Jung Seo, Salahadin Abdi, Billy Huh
Korean J Pain. 2020;33(2):108-120. doi: 10.3344/kjp.2020.33.2.108.
Reference
-
1. Chen XT, Pitis P, Liu G, Yuan C, Gotchev D, Cowan CL, et al. Structure-activity relationships and discovery of a G protein biased µ opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]de can-9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem. 2013; 56:8019–8031. PMID: 24063433.
Article2. Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, et al. Regulation of µ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013; 65:223–254. PMID: 23321159.
Article3. Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003; 63:1256–1272. PMID: 12761335.
Article4. Kochman K. Superfamily of G-protein coupled receptors(GPCRs)--extraordinary and outstanding success of evolution. Postepy Hig Med Dosw (Online). 2014; 68:1225–1237. PMID: 25380205.
Article5. Schiöth HB, Fredriksson R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen Comp Endocrinol. 2005; 142:94–101. PMID: 15862553.
Article6. Smith HS. Peripherally-acting opioids. Pain Physician. 2008; 11:S121–S132. PMID: 18443636.
Article7. Sehgal N, Smith HS, Manchikanti L. Peripherally acting opioids and clinical implications for pain control. Pain Physician. 2011; 14:249–258. PMID: 21587328.8. Burness CB, Keating GM. Oxycodone/naloxone prolonged-release: a review of its use in the management of chronic pain while counteracting opioid-induced constipation. Drugs. 2014; 74:353–375. PMID: 24452879.
Article9. Morlion B, Clemens KE, Dunlop W. Quality of life and healthcare resource in patients receiving opioids for chronic pain: a review of the place of oxycodone/naloxone. Clin Drug Investig. 2015; 35:1–11.
Article10. Violin JD, Crombie AL, Soergel DG, Lark MW. Biased ligands at G-protein-coupled receptors: promise and progress. Trends Pharmacol Sci. 2014; 35:308–316. PMID: 24878326.
Article11. Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999; 286:2495–2498. PMID: 10617462.
Article12. Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000; 408:720–723. PMID: 11130073.
Article13. Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005; 314:1195–1201. PMID: 15917400.14. Soergel DG, Subach RA, Sadler B, Connell J, Marion AS, Cowan CL, et al. First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol. 2014; 54:351–357. PMID: 24122908.
Article15. Soergel DG, Subach RA, Burnham N, Lark MW, James IE, Sadler BM, et al. Biased agonism of the µ-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain. 2014; 155:1829–1835. PMID: 24954166.
Article16. Fossler M, Sadler B, Farrell C, Burt D, Pitsiu M, Skobieranda F, et al. (342) Oliceridine (TRV130), a novel µ receptor G protein pathway selective modulator (µ-GPS), demonstrates a predictable relationship between plasma concentrations and pain relief. I: development of a pharmacokinetic/pharmacodynamic (PK/PD) model. J Pain. 2016; 17:S61.
Article17. Fossler M, Sadler B, Farrell C, Burt D, Pitsiu M, Skobieranda F, et al. (343) Oliceridine (TRV130), a novel µ receptor G protein pathway selective modulator (µ-GPS), demonstrates a predictable relationship between plasma concentrations and pain relief. II: simulation of potential dosing regimens using a pharmacokinetic/pharmacodynamic (PK/PD) model. J Pain. 2016; 17:S61.
Article18. Viscusi E, Minkowitz H, Webster L, Soergel D, Burt D, Subach R, et al. (433) Rapid reduction in pain intensity with oliceridine (TRV130), a novel µ receptor G protein pathway selective modulator (µ-GPS), vs. morphine: an analysis of two phase 2 randomized clinical trials. J Pain. 2016; 17:S82–S83.
Article19. Singla N, Minkowitz H, Soergel D, Burt D, Skobieranda F. (432) Respiratory safety signal with oliceridine (TRV130), a novel µ receptor G protein pathway selective modulator (µ-GPS), vs morphine: a safety analysis of a phase 2b randomized clinical trial. J Pain. 2016; 17:S82.
Article20. Minkowitz H, Singla N, Soergel D, Burt D, Skobieranda F. (435) Nausea and vomiting with oliceridine (TRV130), a novel µ receptor G protein pathway selective modulator (µ-GPS), vs morphine: an analysis of tolerability from a phase 2b randomized clinical trial. J Pain. 2016; 17:S83.
Article21. Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, et al. A randomized, phase 2 study investigating TRV130, a biased ligand of the µ-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016; 157:264–272. PMID: 26683109.
Article22. Madariaga-Mazón A, Marmolejo-Valencia AF, Li Y, Toll L, Houghten RA, Martinez-Mayorga K. Mu-Opioid receptor biased ligands: a safer and painless discovery of analgesics? Drug Discov Today. 2017; 22:1719–1729. PMID: 28743488.
Article23. Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002; 3:639–650. PMID: 12209124.
Article24. Jacoby E, Bouhelal R, Gerspacher M, Seuwen K. The 7 TM G-protein-coupled receptor target family. ChemMedChem. 2006; 1:760–782.25. McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS. G-protein signaling: back to the future. Cell Mol Life Sci. 2005; 62:551–577. PMID: 15747061.
Article26. Patel TB. Single transmembrane spanning heterotrimeric G protein-coupled receptors and their signaling cascades. Pharmacol Rev. 2004; 56:371–385. PMID: 15317909.
Article27. Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011; 17:126–139. PMID: 21183406.
Article28. Kliewer A, Reinscheid RK, Schulz S. Emerging paradigms of G protein-coupled receptor dephosphorylation. Trends Pharmacol Sci. 2017; 38:621–636. PMID: 28478994.
Article29. Dang VC, Christie MJ. Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol. 2012; 165:1704–1716. PMID: 21564086.
Article30. Allouche S, Noble F, Marie N. Opioid receptor desensitization: mechanisms and its link to tolerance. Front Pharmacol. 2014; 5:280. PMID: 25566076.
Article31. Wright JM. The double-edged sword of COX-2 selective NSAIDs. CMAJ. 2002; 167:1131–1137. PMID: 12427705.32. Del Vecchio G, Spahn V, Stein C. Novel opioid analgesics and side effects. ACS Chem Neurosci. 2017; 8:1638–1640. PMID: 28603962.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- N-acetyl-L-cysteine and cysteine increase intracellular calcium concentration in human neutrophils
- The effect of µ-opioid receptor activation on GABAergic neurons in the spinal dorsal horn
- Expression of µ-Opioid Receptor in CA1 Hippocampal Astrocytes
- Tapentadol: Can It Kill Two Birds with One Stone without Breaking Windows?
- Roles of Opioid Receptor Subtype in the Spinal Antinociception of Selective Cyclooxygenase 2 Inhibitor