Korean J Pain.
2016 Jul;29(3):153-157. 10.3344/kjp.2016.29.3.153.
Tapentadol: Can It Kill Two Birds with One Stone without Breaking Windows?
- Affiliations
-
- 1Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Korea. pain@pusan.ac.kr
- KMID: 2327635
- DOI: http://doi.org/10.3344/kjp.2016.29.3.153
Abstract
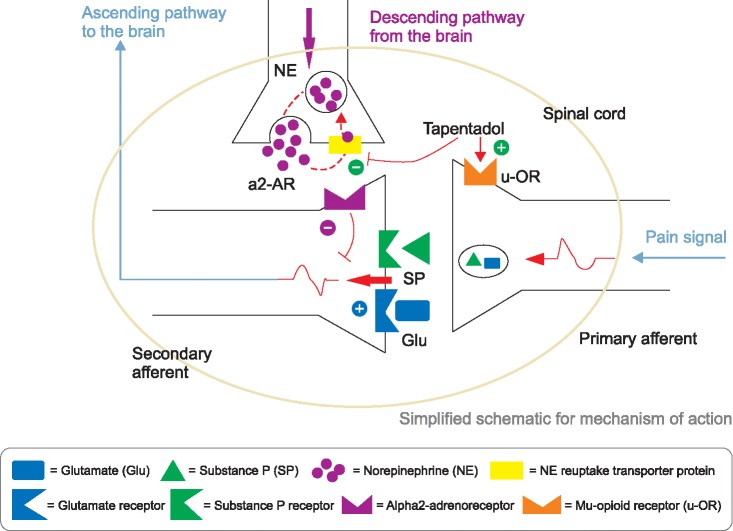
- Tapentadol is a novel oral analgesic with a dual mode of action as an agonist of the µ-opioid receptor (MOR), and as a norepinephrine reuptake inhibitor (NRI) all in a single molecule. Immediate release (IR) tapentadol shows its analgesic effect quickly, at around 30 minutes. Its MOR agonistic action produces acute nociceptive pain relief; its role as an NRI brings about chronic neuropathic pain relief. Absorption is rapid, with a mean maximal serum concentration at 1.25-1.5 h after oral intake. It is present primarily in the form of conjugated metabolites after glucuronidation, and excretes rapidly and completely via the kidneys. The most common adverse reactions are nausea, dizziness, vomiting, and somnolence. Constipation is more common in use of the ER formulation. Precautions against concomitant use of central nervous system depressants, including sedatives, hypnotics, tranquilizers, general anesthetics, phenothiazines, other opioids, and alcohol, or use of tapentadol within 14 days of the cessation of monoamine oxidase inhibitors, are advised. The safety and efficacy have not been established for use during pregnancy, labor, and delivery, or for nursing mothers, pediatric patients less than 18 years of age, and cases of severe renal impairment and severe hepatic impairment. The major concerns for tapentadol are abuse, addiction, seeking behavior, withdrawal, and physical dependence. The presumed problem for use of tapentadol is to control the ratio of MOR agonist and NRI. In conclusion, tapentadol produces both nociceptive and neuropathic pain relief, but with worries about abuse and dependence.
Keyword
MeSH Terms
-
Absorption
Acute Pain
Analgesics, Opioid
Anesthetics, General
Behavior, Addictive
Birds*
Central Nervous System Depressants
Chronic Pain
Constipation
Dizziness
Drug-Related Side Effects and Adverse Reactions
Humans
Hyperalgesia
Hypnotics and Sedatives
Kidney
Monoamine Oxidase Inhibitors
Mothers
Nausea
Neuralgia
Nociceptive Pain
Norepinephrine
Nursing
Phenothiazines
Pregnancy
Receptors, Adrenergic, alpha
Receptors, Opioid, mu
Vomiting
Analgesics, Opioid
Anesthetics, General
Central Nervous System Depressants
Hypnotics and Sedatives
Monoamine Oxidase Inhibitors
Norepinephrine
Phenothiazines
Receptors, Adrenergic, alpha
Receptors, Opioid, mu
Figure
Cited by 1 articles
-
Opioid-induced constipation: a narrative review of therapeutic options in clinical management
Kordula Lang-Illievich, Helmar Bornemann-Cimenti
Korean J Pain. 2019;32(2):69-78. doi: 10.3344/kjp.2019.32.2.69.
Reference
-
1. Lassen D, Damkier P, Brøsen K. The pharmacogenetics of tramadol. Clin Pharmacokinet. 2015; 54:825–836. PMID: 25910878.
Article2. Farquhar-Smith P, Gubbay A. Tramadol and acetaminophen combination for chronic non-cancer pain. Expert Opin Pharmacother. 2013; 14:2297–2304. PMID: 24067074.
Article3. Kress HG. Tapentadol and its two mechanisms of action: is there a new pharmacological class of centrally-acting analgesics on the horizon? Eur J Pain. 2010; 14:781–783. PMID: 20659810.
Article4. Dart RC, Bartelson BB, Adams EH. Nonmedical use of tapentadol immediate release by college students. Clin J Pain. 2014; 30:685–692. PMID: 24042351.
Article5. Dart RC, Cicero TJ, Surratt HL, Rosenblum A, Bartelson BB, Adams EH. Assessment of the abuse of tapentadol immediate release: the first 24 months. J Opioid Manag. 2012; 8:395–402. PMID: 23264317.
Article6. Buschmann H. Chapter 12 Tapentadol – from morphine and tramadol to the discovery of tapentadol. In : Fischer J, Ganellin CR, Rotella DP, editors. Analogue-based drug discovery III. Weinheim: Wiley-VCH;2013. p. 295–318.7. Wade WE, Spruill WJ. Tapentadol hydrochloride: a centrally acting oral analgesic. Clin Ther. 2009; 31:2804–2818. PMID: 20110020.
Article8. Smyth LA, Collins I. Measuring and interpreting the selectivity of protein kinase inhibitors. J Chem Biol. 2009; 2:131–151. PMID: 19568781.
Article9. Tzschentke TM, Folgering JH, Flik G, De Vry J. Tapentadol increases levels of noradrenaline in the rat spinal cord as measured by in vivo microdialysis. Neurosci Lett. 2012; 507:151–155. PMID: 22197547.
Article10. Ramaswamy S, Chang S, Mehta V. Tapentadol--the evidence so far. Anaesthesia. 2015; 70:518–522. PMID: 25866038.11. Ok YM, Cheon JH, Choi EJ, Chang EJ, Lee HM, Kim KH. Nefopam reduces dysesthesia after percutaneous endoscopic lumbar discectomy. Korean J Pain. 2016; 29:40–47. PMID: 26839670.
Article12. Kim KH, Abdi S. Rediscovery of nefopam for the treatment of neuropathic pain. Korean J Pain. 2014; 27:103–111. PMID: 24748937.
Article13. Barber J. Examining the use of tramadol hydrochloride as an antidepressant. Exp Clin Psychopharmacol. 2011; 19:123–130. PMID: 21463069.
Article14. Terlinden R, Ossig J, Fliegert F, Lange C, Göhler K. Absorption, metabolism, and excretion of 14C-labeled tapentadol HCl in healthy male subjects. Eur J Drug Metab Pharmacokinet. 2007; 32:163–169. PMID: 18062408.
Article15. Singh DR, Nag K, Shetti AN, Krishnaveni N. Tapentadol hydrochloride: a novel analgesic. Saudi J Anaesth. 2013; 7:322–326. PMID: 24015138.
Article16. Park CH. Comparison of morphine and tramadol in transforaminal epidural injections for lumbar radicular pain. Korean J Pain. 2013; 26:265–269. PMID: 23862000.
Article17. Xu XS, Smit JW, Lin R, Stuyckens K, Terlinden R, Nandy P. Population pharmacokinetics of tapentadol immediate release (IR) in healthy subjects and patients with moderate or severe pain. Clin Pharmacokinet. 2010; 49:671–682. PMID: 20818833.
Article18. Afilalo M, Etropolski MS, Kuperwasser B, Kelly K, Okamoto A, Van Hove I, et al. Efficacy and safety of Tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clin Drug Investig. 2010; 30:489–505.
Article19. Cepeda MS, Fife D, Ma Q, Ryan PB. Comparison of the risks of opioid abuse or dependence between tapentadol and oxycodone: results from a cohort study. J Pain. 2013; 14:1227–1241. PMID: 23850177.
Article20. Cepeda MS, Fife D, Vo L, Mastrogiovanni G, Yuan Y. Comparison of opioid doctor shopping for tapentadol and oxycodone: a cohort study. J Pain. 2013; 14:158–164. PMID: 23253635.
Article21. Dart RC, Surratt HL, Le Lait MC, Stivers Y, Bebarta VS, Freifeld CC, et al. Diversion and illicit sale of extended release tapentadol in the United States. Pain Med. 2015; pii: pnv032. Forthcoming.
Article22. Butler SF, McNaughton EC, Black RA. Tapentadol abuse potential: a postmarketing evaluation using a sample of individuals evaluated for substance abuse treatment. Pain Med. 2015; 16:119–130. PMID: 25243972.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Can We Kill Two Birds With One Stone? Achieving Function and Aesthetics by Extracorporeal Septoplasty
- 'Killing Two Birds with One Stone' or 'Grasp All, Lose All'
- Two birds with one stone: palonosetron pretreatment
- Molecular Imaging for Theranostics in Gastroenterology: One Stone to Kill Two Birds
- Kill two birds with one stone: selective trunk block (SeTB) with single skin penetration